


 CH3OH(g)��CO��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ����
CH3OH(g)��CO��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ����
 CH3OH(g)��Ӧ��ƽ�ⳣ��Ϊ________(�ú�a��V�Ĵ���ʽ��ʾ)��
CH3OH(g)��Ӧ��ƽ�ⳣ��Ϊ________(�ú�a��V�Ĵ���ʽ��ʾ)��
 CH3OH(g)��H2O(g)�����CO2(g)��CH3OH(g)��Ũ����ʱ��仯��������ͼ��ʾ��
CH3OH(g)��H2O(g)�����CO2(g)��CH3OH(g)��Ũ����ʱ��仯��������ͼ��ʾ��
 CH3OH(g)��Ӧ��ƽ�ⳣ��ΪK="C" (CH3OH)/ { C(CO)��C2(H2)} ="(" a/2V)��{(a/2V) ��(a/V)}2=" V" 2 / a2.��4��CO2(g)+3H2��g��
CH3OH(g)��Ӧ��ƽ�ⳣ��ΪK="C" (CH3OH)/ { C(CO)��C2(H2)} ="(" a/2V)��{(a/2V) ��(a/V)}2=" V" 2 / a2.��4��CO2(g)+3H2��g�� CH3OH(g)+CO(g).��ͼ�ɿ����÷�Ӧ������Ӧ�Ƿ��ȷ�Ӧ���÷�Ӧ������Ӧ�Ǹ����������С�ķ��ȷ�Ӧ�����ԡ�H<0����S<0��ѡ��Ϊ��C����5���ٴӷ�Ӧ��ʼ��ƽ�⣬CO2��ƽ����Ӧ����v(CO2)=��1-0.25��mol/L��10min="0.075" mol/( L��min). ��A�����¶Ȼ�ѧƽ�������ȷ����ƶ������ڸ÷�Ӧ������Ӧ�Ƿ��ȷ�Ӧ���������¶Ȼ�ѧƽ�����淴Ӧ�����ƶ���B����С�������Ũ�ȣ���ѧƽ��������Ӧ�����ƶ����ʽ�CH3OH(g)��ʱҺ���Ƴ���ʹƽ��������Ӧ�����ƶ���C������ �Ի�ѧƽ����Ӱ�졣D���ﵽƽ��ʱ���ٳ���1 mol CO2��3 mol H2����������ѹǿ����ѧƽ�������������С�ķ�������Ӧ�����ƶ���������ʹ��ѧƽ��������Ӧ�����ƶ��Ĵ�ʩ��BD��
CH3OH(g)+CO(g).��ͼ�ɿ����÷�Ӧ������Ӧ�Ƿ��ȷ�Ӧ���÷�Ӧ������Ӧ�Ǹ����������С�ķ��ȷ�Ӧ�����ԡ�H<0����S<0��ѡ��Ϊ��C����5���ٴӷ�Ӧ��ʼ��ƽ�⣬CO2��ƽ����Ӧ����v(CO2)=��1-0.25��mol/L��10min="0.075" mol/( L��min). ��A�����¶Ȼ�ѧƽ�������ȷ����ƶ������ڸ÷�Ӧ������Ӧ�Ƿ��ȷ�Ӧ���������¶Ȼ�ѧƽ�����淴Ӧ�����ƶ���B����С�������Ũ�ȣ���ѧƽ��������Ӧ�����ƶ����ʽ�CH3OH(g)��ʱҺ���Ƴ���ʹƽ��������Ӧ�����ƶ���C������ �Ի�ѧƽ����Ӱ�졣D���ﵽƽ��ʱ���ٳ���1 mol CO2��3 mol H2����������ѹǿ����ѧƽ�������������С�ķ�������Ӧ�����ƶ���������ʹ��ѧƽ��������Ӧ�����ƶ��Ĵ�ʩ��BD��

 ����ʦ��Сһ����ʦ������ҵϵ�д�
����ʦ��Сһ����ʦ������ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ��ѧ�� | H��H | N��H | N��N |
| ����/kJ·mol��1 | 436 | 391 | 945 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A��NaOH(aq)+HCl(aq)��NaCl(aq)+H20(l) =" +28.7" kJ��mol-1 =" +28.7" kJ��mol-1 |
B��NaOH(aq)+HCl(aq)��NaCl(aq)+H20(l) =" -28.7" kJ��mol-1 =" -28.7" kJ��mol-1 |
C��NaOH(aq)+HCl(aq)��NaCl(aq)+H20(l) =" +57.4" kJ��mol-1 =" +57.4" kJ��mol-1 |
D��NaOH(aq)+HCl(aq)��NaCl(aq)+H20(l) =" -57.4" kJ��mol-1 =" -57.4" kJ��mol-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 2NO(g) ��H����֪�÷�Ӧ�� T ��ʱ��ƽ�ⳣ��K��9.0��
2NO(g) ��H����֪�÷�Ӧ�� T ��ʱ��ƽ�ⳣ��K��9.0�� 2NO2(g) ��H1 2NO2(g)
2NO2(g) ��H1 2NO2(g)  O2+2NO(g) ��H2 ��H= ���ú���H1����H2�ı���ʽ��ʾ����
O2+2NO(g) ��H2 ��H= ���ú���H1����H2�ı���ʽ��ʾ����| A������1 mol N2ͬʱ����1 mol O2 |
| B����������ܶȲ��� |
| C���������ƽ����Է����������� |
| D��2v��(N2)��v��(NO) |
 2NO(g)�ġ�K-T������c(NO)-t��ͼ����ͼA������֪�÷�ӦΪ ��Ӧ������ȡ����ȡ�������ͼB��֪����a��Ӧ��������ȣ�b�ı������������ ��
2NO(g)�ġ�K-T������c(NO)-t��ͼ����ͼA������֪�÷�ӦΪ ��Ӧ������ȡ����ȡ�������ͼB��֪����a��Ӧ��������ȣ�b�ı������������ ��
 2NO(g)________________(����ڻ�ѧƽ��״̬������������Ӧ������С������淴Ӧ������С�)��ƽ��ʱ��N2�ڻ�����������ٷ���Ϊ���٣����ڴ����д�����������̣��������2λ��Ч���֣�
2NO(g)________________(����ڻ�ѧƽ��״̬������������Ӧ������С������淴Ӧ������С�)��ƽ��ʱ��N2�ڻ�����������ٷ���Ϊ���٣����ڴ����д�����������̣��������2λ��Ч���֣��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������


 mol;
mol;

 ���>������<����=����
���>������<����=�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 2SO3(g) ��H��0��Ӧ��ij�¶��£�SO2��ƽ��ת���ʣ���������ϵ��ѹǿ��p���Ĺ�ϵ����ͼ��ʾ������ͼʾ�ش��������⣺
2SO3(g) ��H��0��Ӧ��ij�¶��£�SO2��ƽ��ת���ʣ���������ϵ��ѹǿ��p���Ĺ�ϵ����ͼ��ʾ������ͼʾ�ش��������⣺
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��1:3 | B��3:1 | C��1:4 | D��1:1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 CH3OH��H2O����ü״������۲����뷴Ӧ�¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ����ش��������⣺
CH3OH��H2O����ü״������۲����뷴Ӧ�¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ����ش��������⣺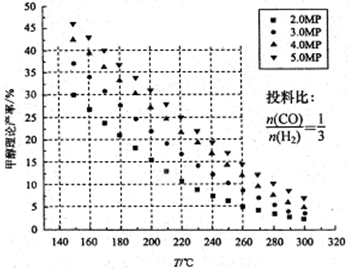
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com