


 ���Ǽ���С����ϵ�д�
���Ǽ���С����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
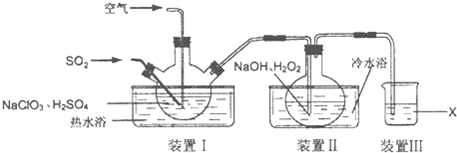
��12�֣�NaClO2�����ޡ��顢ճ����ά��֯���Ư�ס�ʵ�����Ʊ�NaClO2��װ������ͼ��ʾ��
(1)װ��I�����¶���35~55�棬ͨ��SO2��NaClO3��ԭΪClO2���е㣺11�棩����Ӧ������ͨ�������Ŀ�������Ŀ���� ��
(2)װ�â��з�Ӧ����NaClO2�Ļ�ѧ����ʽΪ ����Ӧ�����Һ�������ӳ���ClO2����ClO3����Cl����ClO����OH������ܺ��е�һ���������� ����������ӵķ�����
(3)��֪��NaClO2������Һ���¶ȵ���38��ʱ����������NaClO2��3H2O���¶ȸ���38��ʱ����������NaClO2���¶ȸ���60��ʱNaClO2�ֽ�����NaClO3��NaCl���벹���װ�â�Ӧ�����Һ�л��NaClO2����IJ������衣
�ټ�ѹ��55�������ᾧ���� ���� ���� ���õ���Ʒ��
(4)װ�â����Լ�XΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��12�֣�NaClO2�����ޡ��顢ճ����ά��֯���Ư�ס�ʵ�����Ʊ�NaClO2��װ������ͼ��ʾ��
(1)װ��I�����¶���35~55�棬ͨ��SO2��NaClO3��ԭΪClO2���е㣺11�棩����Ӧ������ͨ�������Ŀ�������Ŀ���� ��
(2)װ�â��з�Ӧ����NaClO2�Ļ�ѧ����ʽΪ ����Ӧ�����Һ�������ӳ���ClO2����ClO3����Cl����ClO����OH������ܺ��е�һ���������� ����������ӵķ�����
(3)��֪��NaClO2������Һ���¶ȵ���38��ʱ����������NaClO2��3H2O���¶ȸ���38��ʱ����������NaClO2���¶ȸ���60��ʱNaClO2�ֽ�����NaClO3��NaCl���벹���װ�â�Ӧ�����Һ�л��NaClO2����IJ������衣
�ټ�ѹ��55�������ᾧ���� ���� ���� ���õ���Ʒ��
(4)װ�â����Լ�XΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ����4��˫����ϰ��ѧ�Ծ��������棩 ���ͣ�ʵ����
��12�֣�NaClO2�����ޡ��顢ճ����ά��֯���Ư�ס�ʵ�����Ʊ�NaClO2��װ������ͼ��ʾ��

(1)װ��I�����¶���35~55�棬ͨ��SO2��NaClO3��ԭΪClO2���е㣺11�棩����Ӧ������ͨ�������Ŀ�������Ŀ���� ��
(2)װ�â��з�Ӧ����NaClO2�Ļ�ѧ����ʽΪ ����Ӧ�����Һ�������ӳ���ClO2����ClO3����Cl����ClO����OH������ܺ��е�һ���������� ����������ӵķ�����
(3)��֪��NaClO2������Һ���¶ȵ���38��ʱ����������NaClO2��3H2O���¶ȸ���38��ʱ����������NaClO2���¶ȸ���60��ʱNaClO2�ֽ�����NaClO3��NaCl���벹���װ�â�Ӧ�����Һ�л��NaClO2����IJ������衣
�ټ�ѹ��55�������ᾧ���� ���� ���� ���õ���Ʒ��
(4)װ�â����Լ�XΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ������ѧ�ڿ�ѧ������⻯ѧ�Ծ� ���ͣ�ʵ����
��12�֣�NaClO2�����ޡ��顢ճ����ά��֯���Ư�ס�ʵ�����Ʊ�NaClO2��װ������ͼ��ʾ��

(1)װ��I�����¶���35~55�棬ͨ��SO2��NaClO3��ԭΪClO2���е㣺11�棩����Ӧ������ͨ�������Ŀ�������Ŀ���� ��
(2)װ�â��з�Ӧ����NaClO2�Ļ�ѧ����ʽΪ ����Ӧ�����Һ�������ӳ���ClO2����ClO3����Cl����ClO����OH������ܺ��е�һ���������� ����������ӵķ�����
(3)��֪��NaClO2������Һ���¶ȵ���38��ʱ����������NaClO2��3H2O���¶ȸ���38��ʱ����������NaClO2���¶ȸ���60��ʱNaClO2�ֽ�����NaClO3��NaCl���벹���װ�â�Ӧ�����Һ�л��NaClO2����IJ������衣
�ټ�ѹ��55�������ᾧ���� ���� ���� ���õ���Ʒ��
(4)װ�â����Լ�XΪ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com