



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��0.10mol��L��1��������Һ����NaCl����NaOH����CH3COONa����pH��С˳��Ϊ���ۣ��ڣ��� |
| B��0.10mol��L��1NaHCO3��Һ�У�c(CO32��)+c(HCO3��)+c(H2CO3)=0.10mol��L��1 |
| C��pH=2�������pH=12��NaOH��Һ�У�ˮ�����ӻ�Kw��ͬ |
| D���������ͬ��pH=2������ʹ�����ȫ��Ӧ����Ҫ0.010mol��L��1 NaOH�������ͬ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| A������ʽ�ζ���ȡϡH2SO4 25.00mL��ע����ƿ�У�����ָʾ���� |
| B���ô��ⶨ����Һ��ϴ��ʽ�ζ��ܡ� |
| C��������ˮϴ�ɾ��ζ��ܡ� |
| D��ȡ�¼�ʽ�ζ����ñ���NaOH��Һ��ϴ����Һע���ʽ�ζ��̶ܿȡ�0������2��3cm�����ٰѼ�ʽ�ζ��̶ܹ��ã�����Һ�����̶ȡ�0����0���̶����¡� |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���÷�̪��ָʾ��ʱ�ζ����ܷ�ӦΪ��Na2CO3+HCl=NaHCO3+NaCl |
| B���ü�����ָʾ��ʱ�ζ����ܷ�ӦΪ��Na2CO3+2HCl=NaCl+CO2��+H2O |
| C�����ü�ʽ�ζ�����ȡ����Ҫ��Na2CO3��Һ |
| D������ʽ�ζ���û���ñ���Һ��ϴ��������õ�̼������ҺŨ��ƫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��12�������� | B��7 | C��6 | D��4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��(10��8��10��10) mol/L | B��(10��4��10��6) mol/L |
| C��(10��8��10��10) mol/L | D��2��10��10 mol/L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
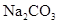 ��Һ�У����е�����ϵ�������ǣ� ��
��Һ�У����е�����ϵ�������ǣ� ��| A��c(OH-)= c(H+)+c(HCO3-)+2c(H2CO3) |
| B��2c(Na+)= c(CO32-)+ c(HCO3-)+ c(H2CO3) |
| C��c(Na+)+ c(H+)= c(HCO3-) +2c(CO32-)+ c(OH-) |
| D��c(Na+)="2" c(CO32-)+ c(HCO3-)+ c(H2CO3) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��pH��ͬʱ�������ʵ���Ũ���ɴ�С��˳���Ǣ�>��>�� |
| B�����ʵ���Ũ����ͬʱ����pH�ɴ�С��˳���Ǣ�>��>�� |
| C���к͵������ռ���Һ��������ʵ���Ũ�ȵĢ٢ڢ���������Һ�������Ϊ2��2��1 |
| D����������ʵ���Ũ�Ⱦ���ͬ�Ģ٢ڢ�����Һ ���ֱ���ͬŨ�ȵ��ռ���Һǡ����ȫ��Ӧ�������ռ���Һ�������Ϊ2��1��2 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com