
ШчЭМЪЕбщзАжУгУгкбщжЄФГаЉЮяжЪЕФаджЪЁЃдкЪдЙмAжазАШызуСПЕФЙЬЬхNaHCO3,DЮЊЙЬЖЈЮУЯуЕФгВжНЦЌЁЃЪдЛиД№ЯТСаЮЪЬтЃК

ЃЈ1ЃЉИУЪЕбщЕФЪЕбщФПЕФЪЧ__________________________________________ЁЃ
ЃЈ2ЃЉдкAЪдЙмФкЗЂЩњЗДгІЕФЛЏбЇЗНГЬЪНЪЧ_______________________________ЁЃ
ЃЈ3ЃЉBзАжУЕФзїгУЪЧ_________________________________________________ЁЃ
ЃЈ4ЃЉдкЫЋЧђИЩдяЙмФкЗЂЩњЗДгІЕФЛЏбЇЗНГЬЪНЮЊ____________________________ЁЃ
ЃЈ5ЃЉЪЕбщЪБЙлВьЕНЕФЪЕбщЯжЯѓЪЧ_______________________________________ЁЃ
ЩЯЪіЪЕбщЯжЯѓЫЕУї___________________________________________________ЁЃ
ЃЈ6ЃЉШєНЋИЩдяЙмФкЕФNa2O2ЛЛГЩNa2OЃЌдђЪЕбщЪБЙлВьЕНЕФЪЕбщЯжЯѓЪЧ___________ЁЃ
ЃЈ1ЃЉбщжЄCO2гыNa2O2ЕФЗДгІ
ЃЈ2ЃЉ2NaHCO3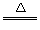 Na2CO3+H2OЁќ+CO2Ёќ
Na2CO3+H2OЁќ+CO2Ёќ
ЃЈ3ЃЉЮќЪеЦјЬхжаЕФЫЎеєЦјЃЈИЩдяCO2ЃЉ
ЃЈ4ЃЉ2Na2O2+2CO2=2Na2CO3+O2
ЃЈ5ЃЉЕЛЦЩЋЕФNa2O2ж№НЅзЊБфЮЊАзЩЋЗлФЉЃЌЕуШМЕФЮУЯуШМЩеИќМгОчСв Na2O2ФмгыCO2ЗДгІЃЌЩњГЩАзЩЋЗлФЉзДЮяжЪКЭO2
ЃЈ6ЃЉЕуШМЕФЮУЯуж№НЅЯЈУ№
ЁОНтЮіЁПИљОнЪЕбщзАжУЭМЃЌПЩжЊИУЪЕбщЪЧбщжЄCO2гыNa2O2ЕФЗДгІЃЌЯШећРэГіЪЕбщЕФВНжшКЭЫГађЮЊЃКAжаЕФNaHCO3ЪмШШЗжНтВњЩњЕФCO2КЭH2OЃЈgЃЉНјШыЪдЙмBЃЌH2OЃЈgЃЉБЛХЈСђЫсЮќЪеЃЌДгBжаГіРДЕФCO2ЦјЬхгыCДІNa2O2ЗДгІЃЌЩњГЩO2ЃЌДгЖјДйНјЮУЯуЕФШМЩеЁЃЕкЃЈ6ЃЉЮЪжаЃЌНЋNa2O2ЛЛГЩNa2OЃЌдђЗЂЩњЗДгІЃКNa2O+CO2=Na2CO3ЃЌЮоO2ЗХГіЃЌЫљвдЮУЯуЛсж№НЅЯЈУ№ЁЃ


 БИеНжаПМКЎМйЯЕСаД№АИ
БИеНжаПМКЎМйЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2014ФъЛЏбЇИпПМзмИДЯАПЮЪБбнСЗ 4-4ЕЊМАЦфживЊЛЏКЯЮяСЗЯАОэЃЈНтЮіАцЃЉ ЬтаЭЃКбЁдёЬт
ЯТСаЫЕЗЈе§ШЗЕФЪЧЃЈ ЃЉ
A.ЕЮМгЯЁNaOHШмвКЃЌНЋЪЊШѓКьЩЋЪЏШяЪджНжУгкЪдЙмПкЃЌЪджНВЛБфРЖЃЌдШмвКжавЛЖЈЮо
B.ЯђзАгаFeЃЈNO3ЃЉ2ШмвКЕФЪдЙмжаМгШыЯЁH2SO4ЃЌПЩдкЙмПкЙлВьЕНКьзиЩЋЦјЬх
C.Й§СПЕФFeЗлжаМгШыЯЁHNO3ЃЌГфЗжЗДгІКѓЃЌЕЮШыKSCNШмвКЃЌШмвКГЪКьЩЋ
D. 46 g NO2КЭN2O4ЛьКЯЦјЬхжаКЌгадзгзмЪ§ЮЊ3NAЃЌКЌгаЕФЗжзгзмЪ§ЮЊNA
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2014ФъЛЏбЇИпПМзмИДЯАПЮЪБбнСЗ 4-1ЮоЛњЗЧН№ЪєВФСЯЕФжїНЧ-ЙшСЗЯАОэЃЈНтЮіАцЃЉ ЬтаЭЃКбЁдёЬт
ОнЁЖВЮПМЯћЯЂЁЗБЈЕРЃЌгаПЦбЇМвЬсГіЙшЪЧЁА21ЪРМЭЕФФмдДЁБЁЂЁАЮДРДЕФЪЏгЭЁБЕФЙлЕуЁЃМйШчЙшзїЮЊвЛжжЦеБщЪЙгУЕФаТаЭФмдДБЛПЊЗЂРћгУЃЌЯТСаЙигкЦфгаРћвђЫиЕФЫЕЗЈжаЃЌФуШЯЮЊВЛЭзЕФЪЧЃЈ ЃЉ
AЃЎБугкдЫЪфЁЂДЂДцЃЌДгАВШЋНЧЖШПМТЧЃЌЙшЪЧзюМбЕФШМСЯ
BЃЎздШЛНчЕФКЌЙшЛЏКЯЮявзПЊВЩ
CЃЎЙшШМЩеЗХГіЕФШШСПДѓЃЌШМЩеВњЮяЖдЛЗОГЮлШОГЬЖШЕЭЧвШнвзгааЇПижЦ
DЃЎздШЛНчжаДцдкДѓСПЕЅжЪЙш
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2014ФъЛЏбЇИпПМзмИДЯАПЮЪБбнСЗ 3-2ТСМАЦфживЊЛЏКЯЮяСЗЯАОэЃЈНтЮіАцЃЉ ЬтаЭЃКбЁдёЬт
НЋ11.9 gгЩMgЁЂAlЁЂFeзщГЩЕФКЯН№ШмгкзуСПЕФNaOHШмвКжаЃЌКЯН№жЪСПМѕЩйСЫ2.7 gЁЃСэШЁЕШжЪСПЕФКЯН№ШмгкЙ§СПЯЁЯѕЫсжаЃЌЩњГЩСЫ6.72 L NOЃЈБъзМзДПіЯТЃЉЃЌЯђЗДгІКѓЕФШмвКжаМгШыЪЪСПNaOHШмвКЧЁКУЪЙMg2+ЁЂAl3+ЁЂFe3+ЭъШЋзЊЛЏЮЊГСЕэЃЌдђГСЕэЕФжЪСПЮЊЃЈ ЃЉ
A.22.1 g B.27.2 g C.30 g D.ЮоЗЈМЦЫу
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2014ФъЛЏбЇИпПМзмИДЯАПЮЪБбнСЗ 3-2ТСМАЦфживЊЛЏКЯЮяСЗЯАОэЃЈНтЮіАцЃЉ ЬтаЭЃКбЁдёЬт
ЯТСаРрБШЙиЯЕе§ШЗЕФЪЧЃЈ ЃЉ
A.AlCl3гыЙ§СПNaOHШмвКЗДгІЩњГЩ ЃЌдђгыЙ§СПNH3ЁЄH2OЗДгІвВЩњГЩ
ЃЌдђгыЙ§СПNH3ЁЄH2OЗДгІвВЩњГЩ
B.Na2O2гыCO2ЗДгІЩњГЩNa2CO3КЭO2ЃЌдђгыSO2ЗДгІПЩЩњГЩNa2SO3КЭO2
C.FeгыCl2ЗДгІЩњГЩFeCl3ЃЌдђгыI2ЗДгІПЩЩњГЩFeI3
D.AlгыFe2O3ФмЗЂЩњТСШШЗДгІЃЌдђгыMnO2вВФмЗЂЩњТСШШЗДгІ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2014ФъЛЏбЇИпПМзмИДЯАПЮЪБбнСЗ 3-1ФЦМАЦфживЊЛЏКЯЮяСЗЯАОэЃЈНтЮіАцЃЉ ЬтаЭЃКбЁдёЬт
ЯТСаИїзщЮяжЪЯрЛЅЛьКЯЗДгІКѓЃЌМШгаЦјЬхЩњГЩЃЌзюжегжгаГСЕэЩњГЩЕФЪЧЃЈ ЃЉ
ЂйН№ЪєФЦЭЖШыЕНFeCl3ШмвКжа
ЂкЙ§СПNaOHШмвККЭУїЗЏШмвКЛьКЯ
ЂлЩйСПCaЃЈOHЃЉ2ЭЖШыЕНЙ§СПNaHCO3ШмвКжа
ЂмNa2O2ЭЖШыFeCl2ШмвКжа
AЃЎжЛгаЂйЂм BЃЎжЛгаЂл CЃЎжЛгаЂкЂл DЃЎжЛгаЂйЂлЂм
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2014ФъЛЏбЇИпПМзмИДЯАПЮЪБбнСЗ 2-3бѕЛЏЛЙдЗДгІСЗЯАОэЃЈНтЮіАцЃЉ ЬтаЭЃКЬюПеЬт
бѕЛЏЛЙдЗДгІдкЩњВњЁЂЩњЛюжаОпгаЙуЗКЕФгУЭОЃЌЙсДЉЙХНёЁЃ
ЃЈ1ЃЉЫЎЪЧШЫЬхЕФживЊзщГЩГЩЗжЃЌЪЧШЫЬхжаКЌСПзюЖрЕФвЛжжЮяжЪЁЃЖјЁАЫФжжЛљБОЗДгІРраЭгыбѕЛЏЛЙдЗДгІЕФЙиЯЕЁБвВПЩгУШчЭМБэДяЁЃ

ЪдаДГігаЫЎВЮМгЕФЗћКЯЗДгІРраЭЂєЕФвЛИіЛЏбЇЗНГЬЪНЃК________________________________ЃЌ
ЦфжаЫЎЮЊ_______МСЁЃ
ЃЈ2ЃЉТШЛЏяЇГЃгУзїКИНгЁЃШчЃКдкКИНгЭЦїЪБгУТШЛЏяЇГ§ШЅЭЦїБэУцЕФбѕЛЏЭвдБуКИНгЃЌЦфЗДгІЮЊЃК
_____CuO+_____NH4Cl _____Cu+_____CuCl2+N2Ёќ+_____H2OЁЃ
_____Cu+_____CuCl2+N2Ёќ+_____H2OЁЃ
ЂйХфЦНДЫбѕЛЏЛЙдЗДгІЗНГЬЪНЁЃ
ЂкИУЗДгІжаЃЌБЛбѕЛЏЕФдЊЫиЪЧ_______ЃЈЬюдЊЫиУћГЦЃЉЃЌбѕЛЏМСЪЧ_______ЃЈЬюЛЏбЇЪНЃЉЁЃ
ЃЈ3ЃЉСзЫсИЦгыНЙЬПЁЂЪЏгЂЩАЛьКЯЃЌдкЕчТЏжаМгШШЕН1 500 ЁцЩњГЩАзСзЃЌЗДгІЮЊЃК2Ca3ЃЈPO4ЃЉ2+6SiO2=6CaSiO3+P4O10 ЃЌ10C+P4O10=P4+10COЁЃУПЩњГЩ1 mol P4 ЪБЃЌОЭга_______molЕчзгЗЂЩњзЊвЦЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2014ФъЛЏбЇИпПМзмИДЯАПЮЪБбнСЗ 2-2РызгЗДгІСЗЯАОэЃЈНтЮіАцЃЉ ЬтаЭЃКбЁдёЬт
ЯТБэжаЦРМлКЯРэЕФЪЧЃЈ ЃЉ
бЁЯюЛЏбЇЗДгІМАЦфРызгЗНГЬЪНЦРМл
AFe3O4гыЯЁЯѕЫсЗДгІЃК2Fe3O4+18H+ =6Fe3++H2Ёќ+8H2Oе§ШЗ
BЯђЬМЫсУОжаМгШыЯЁбЮЫсЃК +2H+ =CO2Ёќ+H2OДэЮѓЃЌЬМЫсУОВЛгІаДГЩРызгаЮЪН
+2H+ =CO2Ёќ+H2OДэЮѓЃЌЬМЫсУОВЛгІаДГЩРызгаЮЪН
CЯђСђЫсяЇШмвКжаМгШыЧтбѕЛЏБЕШмвКЃК
Ba2++ = BaSO4Ё§е§ШЗ
= BaSO4Ё§е§ШЗ
DFeBr2ШмвКгыЕШЮяжЪЕФСПЕФCl2ЗДгІЃК
2Fe2++2Br-+2Cl2=2Fe3++4Cl-+Br2ДэЮѓЃЌFe2+гыBr-ЕФЛЏбЇМЦСПЪ§жЎБШгІЮЊ1ЁУ2
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2014ФъЛЏбЇИпПМзмИДЯАПЮЪБбнСЗ 11-3ЮяжЪЕФжЦБИСЗЯАОэЃЈНтЮіАцЃЉ ЬтаЭЃКбЁдёЬт
ЙЄвЕЩЯгУЯДОЛЕФЗЯЭаМзїдСЯРДжЦБИЯѕЫсЭЁЃЮЊСЫНкдМдСЯКЭЗРжЙЮлШОЛЗОГЃЌвЫВЩШЁЕФЗНЗЈЪЧ( )
A.Cu+HNO3(ХЈ) Cu(NO3)2
Cu(NO3)2
B.Cu CuO
CuO Cu(NO3)2
Cu(NO3)2
C.Cu+HNO3(ЯЁ)  Cu(NO3)2
Cu(NO3)2
D.Cu CuSO4
CuSO4 Cu(NO3)2
Cu(NO3)2
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com