
ij��ɫ��ҺX����Na+��Ag+��Ba2+��Al3+��![]() ��
��![]() ��
��![]() ��
��![]() �е�������������ɣ�ȡ��Һ������������ʵ�飺
�е�������������ɣ�ȡ��Һ������������ʵ�飺
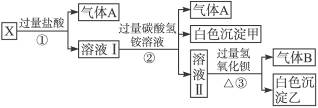
(1)����A�ijɷ���______________������B�ijɷ���_______________��(�ѧʽ)
(2)X��Һ��һ�����ڵ�������_______________��
(3)д��������з�����Ӧ�����е����ӷ���ʽ_______________��
(4)д����������γɰ�ɫ���������ӷ���ʽ_______________��
(5)ͨ������ʵ�飬��ɫ�����ҵ���ɿ�����_______________��ֻҪ���һ���ĺ���ʵ��Ϳ���ȷ���ó�������ɣ��÷�����______________________________��
(1)CO2NH3
(2)Na+��![]() ��
��![]()
(3)C![]() +2H+====CO2��+H2O
+2H+====CO2��+H2O![]() +4H+====Al3++2H2O
+4H+====Al3++2H2O
(4)Al3++3![]() ====Al(OH)3��+3CO2��
====Al(OH)3��+3CO2��
(5)BaCO3��BaCO3��BaSO4�Ļ���� �ڳ������м�������ϡ���ᣬ��ȫ���ܽ���˵��ֻ��BaCO3��������ȫ���ܽ⣬˵����BaCO3��BaSO4�Ļ����
����Һ����ɫ���ų���![]() �ĸ��ţ��ܺ����ᷴӦ�����������
�ĸ��ţ��ܺ����ᷴӦ�����������![]() ��
��![]() �Ĵ����ų���Ag+��Ba2+��Al3+������ԭ��Һ�к��е�������ֻ����Na+����̼�������Һ��Ӧ���ɰ�ɫ����������ֻ����Al3+(��ԭ��Һ�е�
�Ĵ����ų���Ag+��Ba2+��Al3+������ԭ��Һ�к��е�������ֻ����Na+����̼�������Һ��Ӧ���ɰ�ɫ����������ֻ����Al3+(��ԭ��Һ�е�![]() ����������ᷴӦ����)������ԭ��Һ�к���
����������ᷴӦ����)������ԭ��Һ�к���![]() ��������ֻ��ΪAl(OH)3����������ٵķ�ӦΪ��
��������ֻ��ΪAl(OH)3����������ٵķ�ӦΪ��![]() +2H+====CO2��+H2O��
+2H+====CO2��+H2O��![]() +4H+====Al3++2H2O�������ʱ�γɰ�ɫ���������ӷ���ʽΪAl3++3
+4H+====Al3++2H2O�������ʱ�γɰ�ɫ���������ӷ���ʽΪAl3++3![]() ====Al(OH)3��+3CO2����������Ba(OH)2�ṩ�˴�����OH-��Ba2+����������BΪNH3����ɫ�����ҿ�����BaCO3��BaCO3��BaSO4�Ļ�������ȷ�������鷽��Ϊ�ڳ������м�������ϡ���ᣬ��ȫ���ܽ���˵��ֻ��BaCO3��������ȫ���ܽ⣬˵����BaCO3��BaSO4�Ļ���
====Al(OH)3��+3CO2����������Ba(OH)2�ṩ�˴�����OH-��Ba2+����������BΪNH3����ɫ�����ҿ�����BaCO3��BaCO3��BaSO4�Ļ�������ȷ�������鷽��Ϊ�ڳ������м�������ϡ���ᣬ��ȫ���ܽ���˵��ֻ��BaCO3��������ȫ���ܽ⣬˵����BaCO3��BaSO4�Ļ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
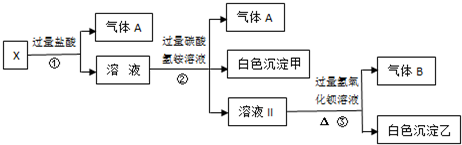
ij��ɫ��ҺX����Na+��Ag+��Ba2+��Al3+��AlO![]() ��MnO
��MnO ��CO
��CO![]() ��SO
��SO![]() �е�������������ɣ�ȡ��Һ������������ʵ�飺
�е�������������ɣ�ȡ��Һ������������ʵ�飺
��1����ɫ�������� ��
��2��X��Һ��һ�����ڵ������� ��
��3����ɫ��������һ���У� �������� ֤�����Ƿ���ڵķ����� ��
��4����������������A������������Bͨ��ˮ�У�д����Ӧ�����ӷ���ʽ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ɫ��ҺX����Na+��Ag+��Ba2+��Al3+��AlO2-��MnO4-��CO32-��SO42-�е�������������ɣ�ȡ��Һ������������ʵ�飺
(1)��ɫ�������� _________��
(2)X��Һ��һ�����ڵ�������_____________��
(3)��ɫ��������һ���У�______��������_______ ֤�����Ƿ���ڵķ�����______________��
(4)��������������A������������Bͨ��ˮ�У�д����Ӧ�����ӷ���ʽ_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ������ʡ������ѧ�ڵ�һ���¿���ѧ�� ���ͣ������
ij��ɫ��ҺX����Na+ ��Ag+ ��Ba2+ ��Al3+ ��[Al(OH)4]-- �� MnO4����CO32-- ��SO42���е�������������ϣ�ȡ��Һ���������������飺���ѧ���
 1������A�ijɷ��ǣ�_________________������B�ijɷ���_____________
1������A�ijɷ��ǣ�_________________������B�ijɷ���_____________
2)X��Һ��һ�����ڵ������ǣ�____________________________
3)д������ٷ�����Ӧ���������ӷ�Ӧ����ʽ��L_________________________
4��д��������γɰ�ɫ���������ӷ���ʽ��______________________
5)д����ɫ�����ҵĿ�����ɣ�____________________________
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com