
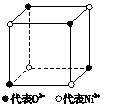
��1����ѧ�̲���ͼʾ��NaCl����Ľṹ��������ά�ռ������γ��������塣��������NiO������Ľṹ��NaCl��ͬ��Ni2+�����ڽ���O2-�˼����Ϊa��10-8 cm������NiO������ܶȣ���֪NiO��Ħ������Ϊ74.7 g�� mol-1����
��2����Ȼ�ĺ;����˹��Ʊ��ľ��嶼���ڸ���ȱ�ݣ�������ij��NiO�����оʹ�������ͼ��ȱ�ݣ�һ��Ni2+��ȱ����������Ni2+������Ni3+��ȡ�������������Գʵ����ԣ�����������Ni��O�ĸ�����ȴ�����˱仯��ij��������Ʒ�����ΪNi0.97O���Լ���þ�����Ni3+��Ni2+��������֮�ȡ�

��1��![]() g��cm-3 ��2��6��91
g��cm-3 ��2��6��91
��1��ѡ�����µ�һ��С�����������м��㣺
��ͳ�ƵĽǶȿ�����С��������е�Ni2+��O2-���Ӷ���Ϊ0.5��
�������������![]() ��0.5=6.20��10-23 g
��0.5=6.20��10-23 g
��������������a��10-8 cm )3=a3��10-24 cm3
С��������ܶ�Ϊ��![]() =
=![]()
��2����1 mol Ni0.97O�к�Ni3+�����ʵ���Ϊx����Ni2+�����ʵ���Ϊ0.97��x��
���ݾ���ʵ����Կ��г���3x+2(0.97��x)=2�����x=0.06
��������֮��Ϊ��Ni3+��Ni2+=0.06��(0.97��0.06)=6��91


 �����ҵ���������ͯ������ϵ�д�
�����ҵ���������ͯ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��1����ѧ�̲���ͼʾ��NaCl����ṹ��������ά�ռ�����õ��������壮 NiO��������������Ľṹ��NaQ��ͬ��Ni2+�����ڽ�O2-�ĺ˼����Ϊa10-8cm������NiO������ܶȣ���֪NiO��Ħ������Ϊ74.7g��mol-1����
��1����ѧ�̲���ͼʾ��NaCl����ṹ��������ά�ռ�����õ��������壮 NiO��������������Ľṹ��NaQ��ͬ��Ni2+�����ڽ�O2-�ĺ˼����Ϊa10-8cm������NiO������ܶȣ���֪NiO��Ħ������Ϊ74.7g��mol-1�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
NiO��������������Ľṹ��NaCl��ͬ��Ni2+�����ڽ�O2���ĺ˼����Ϊa��10��8 cm������NiO������ܶȡ�����֪NiO��Ħ������Ϊ74.7 g��mol��1��
��2����Ȼ�ĺ;����˹��Ʊ��ľ��嶼����ȱ�ݣ�������ij��NiO�����оʹ�����ͼ��ʾ��ȱ�ݣ�һ��Ni2+��ȱ����������Ni2+������Ni3+��ȡ���������Ǿ����Գʵ����ԣ�����������Ni��O�ı�ֵȴ�����˱仯��ij��������Ʒ���ΪNi0.97O���Լ���þ�����Ni3+��Ni2+��������֮�ȡ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��2����Ȼ�ĺ;����˹��Ʊ��ľ��嶼���ڸ���ȱ�ݣ�������ij��NiO�����оʹ�������ͼ��ȱ�ݣ�һ��Ni2+��ȱ����������Ni2+������Ni3+��ȡ�������������Գʵ����ԣ�����������Ni��O�ĸ�����ȴ�����˱仯��ij��������Ʒ�����ΪNi0.97O���Լ���þ�����Ni3+��Ni2+��������֮�ȡ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

��2����Ȼ�ĺ;����˹��Ʊ��ľ��嶼���ڸ���ȱ�ݣ�������ij��NiO�����оʹ�����ͼ��ȱ�ݣ�һ��Ni2+��ȱ����������Ni2+������Ni3+��ȡ�������������Գʵ����ԣ�����������Ni��O�ı�ֵȴ�����˱仯��ij��������Ʒ�����ΪNi0.97O���Լ���þ�����Ni3+��Ni2+��������֮�ȡ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com