
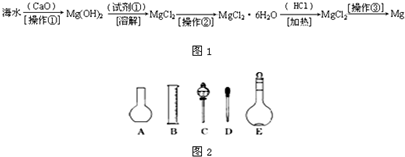
| n |
| V |
| ||



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ɽ��ʡ�ij���ij�ص���ѧ��һ���Ĵ�ģ���⻯ѧ�Ծ����������� ���ͣ�ʵ����
��һ�������е����������ʣ�д����ȥ��Щ���ʵ��Լ���
��1��MgO (Al2O3) ��2��Cl2(HCl) �� ��
��3��FeCl3(FeCl2) �� ��4��NaHCO3��Һ(Na2CO3) ��
������(6��)��ˮ�к��д������Ȼ�þ���Ӻ�ˮ����ȡþ��������������ͼ��ʾ��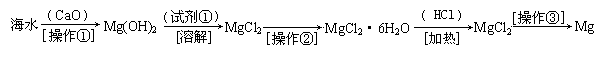
�ش��������⣺
д���ں�ˮ�м�������������������þ�Ļ�ѧ����ʽ���������� ����������������
��������Ҫ��ָ �� �� ���Լ��ٿ�ѡ�� �� �� ��
��������ָ�������� �������� �������������տɵý���þ��
��������8�֣�ʵ��������480ml 0.1mol��L-1��Na2CO3��Һ���ش��������⣺
��1��Ӧ��������ƽ��ȡʮˮ̼���ƾ��� g��
��2����ͼ��ʾ������������Һ�϶�����Ҫ������ �� (�����)����ʵ�����貣������E���Ϊ���� ����mL��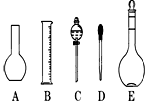
��3������ƿ�ϱ��У����¶ȡ���Ũ�ȡ�����������ѹǿ���ݿ̶��ߡ�����ʽ���ʽ�������е� ���������ַ��ţ�
��4�������������Ҫ�����ǣ�a����ƿ��b�ձ���c��ͷ�ιܡ�d������ƽ�������ڲ���������ʹ�õ�ǰ��˳���� ������д��ĸ��ÿ������ֻ��ѡ��һ�Σ�
��5���������ǻ�ѧʵ���г��õ�һ�ֲ������ߣ�����������Һ�Ĺ����в�����������
����;������д���֣�
��6����ʵ��ʱ���������������ʹ��Һ��Ũ��ƫ�͵��� ��
| A������ǰû�н�����ƿ�е�ˮ������ | B��̼����ʧȥ�˲��ֽᾧˮ�� |
| C��̼���ƾ��岻�������л����Ȼ��ƣ� | D������̼���ƾ���ʱ�����������⣻ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015��ɽ��ʡ�ij��и�һ���Ĵ�ģ���⻯ѧ�Ծ��������棩 ���ͣ�ʵ����
��һ�������е����������ʣ�д����ȥ��Щ���ʵ��Լ���
��1��MgO (Al2O3) ��2��Cl2(HCl) �� ��
��3��FeCl3(FeCl2) �� ��4��NaHCO3��Һ(Na2CO3) ��
������(6��)��ˮ�к��д������Ȼ�þ���Ӻ�ˮ����ȡþ��������������ͼ��ʾ��
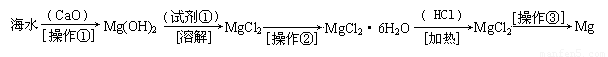
�ش��������⣺
д���ں�ˮ�м�������������������þ�Ļ�ѧ����ʽ���������� ����������������
��������Ҫ��ָ �� �� ���Լ��ٿ�ѡ�� �� �� ��
��������ָ�������� �������� �������������տɵý���þ��
��������8�֣�ʵ��������480ml 0.1mol��L-1��Na2CO3��Һ���ش��������⣺
��1��Ӧ��������ƽ��ȡʮˮ̼���ƾ��� g��
��2����ͼ��ʾ������������Һ�϶�����Ҫ������ �� (�����)����ʵ�����貣������E���Ϊ���� ����mL��
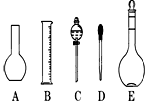
��3������ƿ�ϱ��У����¶ȡ���Ũ�ȡ�����������ѹǿ���ݿ̶��ߡ�����ʽ���ʽ�������е� ���������ַ��ţ�
��4�������������Ҫ�����ǣ�a����ƿ��b�ձ���c��ͷ�ιܡ�d������ƽ�������ڲ���������ʹ�õ�ǰ��˳���� ������д��ĸ��ÿ������ֻ��ѡ��һ�Σ�
��5���������ǻ�ѧʵ���г��õ�һ�ֲ������ߣ�����������Һ�Ĺ����в�����������
����;������д���֣�
��6����ʵ��ʱ���������������ʹ��Һ��Ũ��ƫ�͵��� ��
A������ǰû�н�����ƿ�е�ˮ������ B��̼����ʧȥ�˲��ֽᾧˮ��
C��̼���ƾ��岻�������л����Ȼ��ƣ� D������̼���ƾ���ʱ�����������⣻
E. ����ʱ���ӿ̶���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
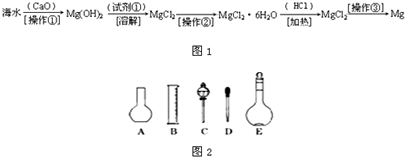
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ɽ��ʡ�ij�����У��һ���ϣ���ĩ��ѧ�Ծ��������棩 ���ͣ������
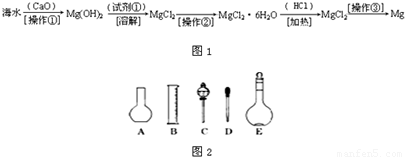
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com