
����Ŀ��1molij��A��1 mol����ȫȼ�գ�����ͬ�����£�����CO2�����ͬ����A�ȱ�������1.5mol O2���Իش�
��1����A�ķ���ʽ��__________ ��
��2������A����ʹ��ˮ��ɫ������һ������������Cl2����ȡ����Ӧ����һ�ȴ���ֻ��һ�֣�����A�Ľṹ��ʽΪ_______________ ��
��3������A��ʹ��ˮ��ɫ���ҷ���������̼ԭ�ӹ�ƽ�棬��A�Ľṹ��ʽΪ____________________________ ��
��4����A��2��̼ԭ�ӵ�A��ϩ����ͬϵ���ͬ���칹�壨���������칹������__________�֡�
���𰸡� C6H12 ![]() ��CH3��2C=C��CH3��2 4
��CH3��2C=C��CH3��2 4
�����������������������Ҫ�����л������ɡ��ṹ�����ʡ�
1molij��A��1 mol����ȫȼ�գ�����ͬ�����£�����CO2�����ͬ��˵��A�ͱ��ķ��Ӷ�����6��̼ԭ�ӣ���A�ȱ�������1.5mol O2��˵��A���ӱȱ����Ӷ�6����ԭ�ӣ����A����ʽΪC6H12��
��1����A�ķ���ʽ��C6H12��
��2������A����ʹ��ˮ��ɫ��˵��Aû�в����ͽṹ������һ������������Cl2����ȡ����Ӧ����һ�ȴ���ֻ��һ�֣�˵��A�ķ��ӽṹ�߶ȶԳƣ�����A�Ľṹ��ʽΪ![]() ��
��
��3������A��ʹ��ˮ��ɫ��˵��A����̼̼˫�����������ҷ���������̼ԭ�ӹ�ƽ�棬˵������˫��̼ԭ�ӻ���˫��̼ԭ��������̼ԭ�ӣ���A�Ľṹ��ʽΪ��CH3��2C=C��CH3��2��
��4����A��2��̼ԭ�ӵ�A��ϩ����ͬϵ��ķ���ʽΪC4H8��ͬ���칹�壨���������칹����1-��ϩ��˳ʽ2-��ϩ����ʽ2-��ϩ��2-��-1-��ϩ��4�֡�


 ���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ĺ���������B����Է�������Ϊ180������̼Ԫ�ص���������Ϊ60%����Ԫ�ص���������Ϊ35.6%����ش��������⣺
(1)B�ķ���ʽΪ_________________��
(2)B����λ��ȡ����������һ��ȡ�������Ȼ���B�ܷ�����ͼ��ʾת����

��C�� E�ķ�Ӧ��ϵ�к�18O��������_________�֡�
��D������Ũ��ˮ��Ӧ����Ҫ����Ľṹ��ʽΪ__________________________________��
��F����������Щ��ѧ���ʣ�____________��
A����Ũ��ˮ��Ӧ B�������ӳɷ�Ӧ C������������Ӧ D. ������ȥ��Ӧ
��B������NaOH��Һ���ȵĻ�ѧ����ʽΪ��
________________________________________________________________________��
C��һ��ͬ���칹�岻�ܷ���ˮ�ⷴӦ�����ܷ���������Ӧ����������Ӧ�ķ���ʽΪ��_________________________________________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��25 ��ʱ����Ũ��Ϊ0.100 0 mol��L��1��NaOH��Һ�ֱ�ζ�20.00 mLŨ�Ⱦ�Ϊ0.100 0 mol��L��1��HX��HY��Һ��pH��NaOH��Һ����ı仯��ͼ��ʾ������˵����ȷ����( )

A. ��V(NaOH)��0 mLʱ��c(X��)>c(Y��)
B. a��ʱ��c(Y��)��c(HY)��0.100 0 mol��L��1
C. b��ʱ��c(HX)>c(Na��)>c(X��)>c(OH��)>c(H��)
D. ��V(NaOH)��20.00 mLʱ��NaX��NaY����Һ�е�������������Դ�С��Nǰ��>N����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������1 L 0.1 mol��L��1 NH4Cl��Һ�������ϼ������NaOH����NH![]() ��NH3 �� H2O�ı仯��������ͼ��ʾ(����������仯�Ͱ��Ļӷ�)������˵������ȷ����(����)
��NH3 �� H2O�ı仯��������ͼ��ʾ(����������仯�Ͱ��Ļӷ�)������˵������ȷ����(����)
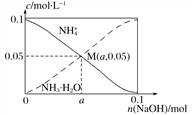
A. ��M��ʱ��n(OH��)��n(H��)��(a��0.05) mol
B. ����NaOH�ļ�����![]() ��������
��������
C. M����Һ��ˮ�ĵ���̶ȱ�ԭ��ҺС
D. ��n(NaOH)��0.1 molʱ��c(OH��)��c(Cl��)��c(NH3��H2O)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��DAHBA��ij�{�ºϳɲ��ϵ�һ�ֵ��壬��������ASA������ˮ���ᣩΪԭ���Ʊ�DAHBA��һ�ֺϳ�·�ߣ�

�ش��������⣺
��1��ASA���ԵĹ�������_____________________��д���ƣ���
��2��AHNBA�Ľṹ��ʽΪ_______________________________��
��3����Ӧ�ڵķ�Ӧ����Ϊ___________________��
��4����Ӧ�١��۵�Ŀ����__________________________________��
��5����Ӧ�ٵĻ�ѧ����ʽ__________________________________��
��6��ˮ���ᣨ ����ͬ���칹���У�����FeCl3��Һ������ɫ��Ӧ���ܷ���������Ӧ�Ĺ���_____________ �֣������ܷ���ˮ�ⷴӦ�Һ˴Ź���������ʾ��4���Ľṹ��ʽΪ_______________________��
����ͬ���칹���У�����FeCl3��Һ������ɫ��Ӧ���ܷ���������Ӧ�Ĺ���_____________ �֣������ܷ���ˮ�ⷴӦ�Һ˴Ź���������ʾ��4���Ľṹ��ʽΪ_______________________��
��7��д���Լױ�Ϊԭ�ϣ����Լ���ѡ���Ʊ�2��4��6-������������ĺϳ�·�ߣ�_____________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NAΪ�����ӵ�������ֵ������˵���������
A. ��״���£�22.4L NO��11.2 LO2��ַ�Ӧ���������С��NA
B. 1molNa2O2������ˮ��Ӧת�Ƶ�����ΪNA
C. ��2molH2SO4��Ũ����������Cu��Ӧ������NA��SO2����
D. ���³�ѹ�£�28g N2��CO�Ļ�������к���ԭ�ӵ���Ŀ����2NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ȼ����ʯͨ������Fe2O3����������ҵ���ô���ʯ��ԭ��ʱ��Ҫ�ᴿ��ij�о���ѧϰС������ʦ��ָ�������Դ���ʯ�������ᴿ����������µ�ʵ��������

��ش�����������
(1)�ܽ����ʯʱ������������������ԭ����____________________________________________��
(2)��������������________���ò������õ����������������ڸ�ʵ���е�������___________________________��
(3)������ҺB���Ƿ������ӵIJ���������_________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й��������飨b����2-�����飨d�����ױ���p����˵������ȷ����
A. b��d��Ϊͬϵ��
B. b��d��p��һ�ȴ��������ֱ�Ϊ2��3��4
C. b��d��p������ʹ���Ը��������Һ��ɫ
D. b��d��p��ֻ��d������̼ԭ�Ӳ��ܴ���ͬһƽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������Ͷ��һ��Ũ�ȵ��Ȼ������Ȼ�ͭ�Ļ����Һ�У���ַ�Ӧ����Һ��ʣ������������������۵�������ͬ����ԭ��Һ���Ȼ������Ȼ�ͭ��Ũ��֮��Ϊ
A��2��7 B��3��4 C��4��7 D��2��3
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com