
����0.8 mol��HNO3��ϡ������Һ����������22.4 g�����ۣ����跴Ӧ��Ϊ�����Σ���һ��Ϊ��Fe��HNO3��Fe(NO3)3��NO����H2O
(1)д���������η�Ӧ�����ӷ���ʽ��
(2)���������η�Ӧ�У��������۵����ʵ�������Һ����Ԫ�ش��ڵ���ʽ��
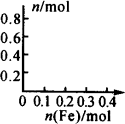
(3)��ͼ�л�����Һ��Fe2+��NO3�����ӵ����ʵ�����������۵����ʵ����仯�Ĺ�ϵͼ��(������Ϊ�������۵����ʵ���������������Һ�����ӵ����ʵ���)��
 ��ս100��Ԫ����Ծ�ϵ�д�
��ս100��Ԫ����Ծ�ϵ�д� ������ϵ�д�
������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2013�찲��ʡ�������и����������¿���ѧ�Ծ����������� ���ͣ������
(10��)����0.8 mol��ϡ��������������22.4 g�����ۣ����跴Ӧ��Ϊ�����Ρ�
��һ��Ϊ��Fe��HNO3(ϡ)�D��Fe(NO3)3��NO����H2O
��1��д���������η�����Ӧ�����ӷ���ʽ��
��һ�Σ� ��
�ڶ��Σ� ��
��2����ȷ�����������η�Ӧ�У��������۵����ʵ�������Һ����Ԫ�ش��ڵ���ʽ�Ĺ�ϵ
��
��3����ͼ�л�����Һ��Fe2����Fe3����NO�����ʵ�����������۵����ʵ����仯�Ĺ�ϵͼ��(������Ϊ�������۵����ʵ���������������Һ�����ӵ����ʵ���)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�����ʡ֣���и�����һ������Ԥ�⻯ѧ�Ծ��������棩 ���ͣ������
(8��)ʵ������ NaHSO4 ,Ba(OH)2, NH3 ? H2O NaHCO3��KAl(SO4)2������ɫ��Һ������ͨ������֮������Ӧ���������м��𡣲������ʼ�ķ�Ӧ�������±���
���С� ����ʾ�����������ʣ���
����ʾ�����������ʣ��� ����ʾ���ɳ�����
����ʾ���ɳ�����
����������Ϣ���ش��������⣺
(1)B��E�Ļ�ѧʽ�ֱ�Ϊ_______��___________��
(2) д��A�ĵ��뷽��ʽ___________________________����
(3) C��D����Һ��Ӧ�����ӷ���ʽΪ___________________________��
(4) ����0.1 mol���ʵ�D��Һ�еμ�E��Һ�������ɳ��������ʵ���֮�����Ϊ_________mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�갲��ʡ�����������¿���ѧ�Ծ��������棩 ���ͣ������
(10��)����0.8 mol��ϡ��������������22.4 g�����ۣ����跴Ӧ��Ϊ�����Ρ�
��һ��Ϊ��Fe��HNO3(ϡ)�D��Fe(NO3)3��NO����H2O
��1��д���������η�����Ӧ�����ӷ���ʽ��
��һ�Σ� ��
�ڶ��Σ� ��
��2����ȷ�����������η�Ӧ�У��������۵����ʵ�������Һ����Ԫ�ش��ڵ���ʽ�Ĺ�ϵ
��
��3����ͼ�л�����Һ��Fe2����Fe3����NO�����ʵ�����������۵����ʵ����仯�Ĺ�ϵͼ��(������Ϊ�������۵����ʵ���������������Һ�����ӵ����ʵ���)��

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com