
���������( Na2S2O3)�׳Ʊ��շۣ�����������ҵ����Ӱ����Ҳ������ֽ��Ư�����������ȡ�ʵ���ҿ�ͨ�����·�Ӧ��ȡ��2Na2S+Na2CO3+4SO2=3Na2S2O3+CO2��
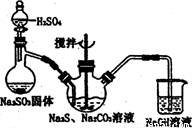
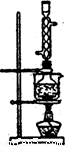
ͼl ͼ2
(1)��ͼl��ʾװ����ȡNa2S2O3������NaOH��Һ�������ǣ� ��
�罫��Һ©���е�H2SO4�ij�Ũ���ᣬ��������ƿ�ڳ�Na2S2O3�����⣬���� (�ѧʽ)�������ɡ�
Ϊ�ⶨ���ñ������Ʒ��Na2S2O3��5H2O���������������ñ�����Һ���еζ�����Ӧ����ʽΪ2 Na2S2O3+I2=2NaI+Na2S4O6��
(2)����KIO3��KI��HCI�����Ʊ�����Һ��д������ʱ��������Ӧ�����ӷ���ʽ�� ��
(3)ȷ��ȡһ��������Na2S2O3��5H2O��Ʒ����ƿ�У���ˮ�ܽ⣬���μ� ��ָʾ�����������Ƶı�����Һ�ζ����ζ�ʱ���õIJ�����������ƿ�⣬���� ��
(4)���춨ʱ����֣��տ�����Һ�ֲ���ɫ��ֹͣ�춨�����ʹ��Ʒ��Na2S2O3��5H2O�����������IJ������____(�ƫ�ߡ�ƫ�͡����䡱)��
(5)��ʵ���Na2S�Ĵ���Ҫ��ϸߣ�����ͼ2��ʾ��װ�ÿɽ���ҵ����Na2S�ᴿ��
��֪Na2S���������ھƾ�������ʱ�ܽ��Ѹ���������ʲ����ھƾ����ᴿ��������Ϊ��
�ٽ��ѳ����õĹ�ҵNa2S����Բ����ƿ�У�������һ�������ľƾ�������ˮ��
�ڰ�ͼ2��ʾװ����������������������ͨ����ȴˮ��ˮԡ���ȣ�
�۴���ƿ�й��岻�ټ���ʱ��ֹͣ���ȣ�����ƿȡ�¡�
�� ��
�� ��
�����ù���ϴ�ӡ�����õ�Na2S��9H2O���塣
��1������SO2��β������ֹ��Ⱦ���� NaCl
��2��IO3-+5I-+6H+=3I2+3H2O
��3��������Һ �ζ���
��4�����ȹ��� ��������Һ��ȴ�ᾧ������
��������
���������
��1������NaOH����β������ֹ��Ⱦ���� �����ij����ᣬ���������NaCl��
��2��IO3-��I-�����������·������з�Ӧ��
��3������ʹ�õ�����ָʾ�����ζ������õ��ζ��ܡ�
��4���ֲ���ɫ˵����Ӧδ��ȫ�����ٵμ��˱�Һ��ʹ�����������ƫ�͡�
��5��Na2S������ʱ�ܽ�ȴ����Ҫ���ȹ��ˡ�
���㣺���⿼����ʵ��Ļ���������������ԭ��Ӧ����д���к͵ζ���֪ʶ��


 ��Ӣ���㿨ϵ�д�
��Ӣ���㿨ϵ�д� Ӧ����㲦ϵ�д�
Ӧ����㲦ϵ�д� ״Ԫ����ϵ�д�
״Ԫ����ϵ�д� ͬ������ϵ�д�
ͬ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| 0.496v |
| m |
| 0.496v |
| m |
| ʵ����� | ��ҺpH | ����������ˮ�������� | ��Ӧ�¶� | ������� | ��������ת���� |
| 1 | 10 | 1.5��1 | 100 | 18 | 80.7% |
| 2 | a | 1.1��1 | 100 | 18 | 94.6% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ӱ�ʡʯ��ׯ�е�һ��ѧ��һ��ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ���ѡ��
��������ƣ�Na2S2O3����ϡH2SO4��Һ���ʱ�������·�Ӧ��
Na2S2O3+ H2SO4=Na2 SO4+SO2+S��+H2O���з�Ӧ����������
| A��0.1mol/L Na2S2O3��0.1mol/L H2SO4��Һ��5mL����ˮ5mL����Ӧ�¶�10�� |
| B��0.1mol/L Na2S2O3��0.1mol/L H2SO4��Һ��5mL����ˮ10mL����Ӧ�¶�10�� |
| C��0.1mol/L Na2S2O3��0.1mol/L H2SO4��Һ��5mL����ˮ10mL����Ӧ�¶�30�� |
| D��0.2mol/L Na2S2O3��0.1mol/L H2SO4��Һ��5mL����ˮ10mL����Ӧ�¶�30�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��κ�һ�и�����һ�������Ի�ѧ�� ���ͣ�ʵ����
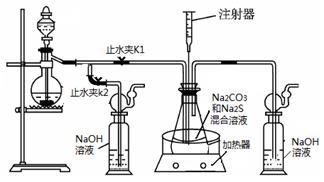
��16�֣����ƺ�̼����Ϊԭ�ϡ���������װ���Ʊ���������ƣ��Ʊ���Ӧ�ɱ�ʾΪ��
2Na2 S +Na2CO3 + 4SO2  3Na2S2O3 +CO2������Ҫ��ش����⣺
3Na2S2O3 +CO2������Ҫ��ش����⣺
��1��ʵ��ʱ����K1���ر�K2�����ϴ��ƿ�з�����Ӧ�����ӷ���ʽ�� ��
��2����ƿ����ҺpHС��7ʱ�ᵼ�²�Ʒ�ֽ⣬���ʵ���������Ҫ������ƿ����Һ��pH��
�ٷ�Ӧ�����У���ƿ����ҺpH��________�����������С�����ֲ��䡱����
�ڲ�����ƿ����ҺpHʱ����ע������ȡ��Һ��Ʒ��ֱ�Ӵ���ƿ��ƿ��ȡ��������������⣬�����е��ŵ��� ��
����ʵ������в����ҺpH�ѽӽ���7����ʱӦ�ý��еIJ����� ��
��3����֪��2Na2 S2O3 +I2="2NaI+" Na2 S4O6��ʵ������������ش������ɼ����Na2 S2O3 ��5H2O���塣Ϊ�����䴿�ȣ�ȡ������Ʒmg����ˮ�ܽ���뼸�ε�����Һ����0��010mol/L��ˮ�ζ����յ�ʱ�����ĵ�ˮ��ҺvmL�������Ʒ������ ��
��4����ȡ��������Ƶ���һ�ַ�����ֱ�ӽ���ۺ��������ơ�ˮ��Ϲ�����ȡ��Ϊ̽����ȡ��������������������ҺpH����������Ũ�ȡ���Ӧ�¶ȡ������������������¶Ա�ʵ�飨ÿ��ʵ��ʱ��������������Ϊ63g����Ӧʱ��Ϊ30min����
| ʵ����� | ��ҺpH | ����������ˮ�������� | ��Ӧ�¶� | ������� | ��������ת���� |
| 1 | 10 | 1��5��1 | 100 | 18 | 80��7% |
| 2 | a | 1��1��1 | 100 | 18 | 94��6% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015��ӱ�ʡʯ��ׯ�и�һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��������ƣ�Na2S2O3����ϡH2SO4��Һ���ʱ�������·�Ӧ��
Na2S2O3+ H2SO4=Na2 SO4+SO2+S��+H2O���з�Ӧ����������
A��0.1mol/L Na2S2O3��0.1mol/L H2SO4��Һ��5mL����ˮ5mL����Ӧ�¶�10��
B��0.1mol/L Na2S2O3��0.1mol/L H2SO4��Һ��5mL����ˮ10mL����Ӧ�¶�10��
C��0.1mol/L Na2S2O3��0.1mol/L H2SO4��Һ��5mL����ˮ10mL����Ӧ�¶�30��
D��0.2mol/L Na2S2O3��0.1mol/L H2SO4��Һ��5mL����ˮ10mL����Ӧ�¶�30��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
���ƺ�̼����Ϊԭ�ϡ���������װ���Ʊ���������ƣ��Ʊ���Ӧ�ɱ�ʾΪ��
2Na2 S +Na2CO3 + 4SO2 ![]() 3Na2S2O3 +CO2������Ҫ��ش����⣺
3Na2S2O3 +CO2������Ҫ��ش����⣺
��1��ʵ��ʱ����K1���ر�K2�����ϴ��ƿ�з�����Ӧ�����ӷ���ʽ�� ��
��2����ƿ����ҺpHС��7ʱ�ᵼ�²�Ʒ�ֽ⣬���ʵ���������Ҫ������ƿ����Һ��pH��
�ٷ�Ӧ�����У���ƿ����ҺpH��________�����������С�����ֲ��䡱����
�ڲ�����ƿ����ҺpHʱ����ע������ȡ��Һ��Ʒ��ֱ�Ӵ���ƿ��ƿ��ȡ��������������⣬�����е��ŵ��� ��
����ʵ������в����ҺpH�ѽӽ���7����ʱӦ�ý��еIJ����� ��
��3����֪��2Na2 S2O3 +I2=2NaI+ Na2 S4O6��ʵ������������ش������ɼ����Na2 S2O3 ��5H2O���塣Ϊ�����䴿�ȣ�ȡ������Ʒmg����ˮ�ܽ���뼸�ε�����Һ����0��010mol/L��ˮ�ζ����յ�ʱ�����ĵ�ˮ��ҺvmL�������Ʒ������ ��
��4����ȡ��������Ƶ���һ�ַ�����ֱ�ӽ���ۺ��������ơ�ˮ��Ϲ�����ȡ��Ϊ̽����ȡ��������������������ҺpH����������Ũ�ȡ���Ӧ�¶ȡ������������������¶Ա�ʵ�飨ÿ��ʵ��ʱ��������������Ϊ63g����Ӧʱ��Ϊ30min����
| ʵ����� | ��ҺpH | ����������ˮ�������� | ��Ӧ�¶� | ������� | ��������ת���� |
| 1 | 10 | 1��5��1 | 100 | 18 | 80��7% |
| 2 | a | 1��1��1 | 100 | 18 | 94��6% |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com