

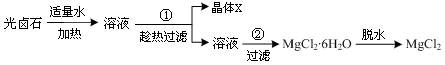
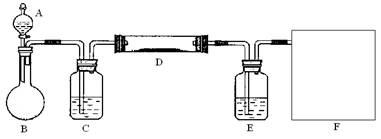
 МмМмПтЙПТ»±ѕєГѕнПµБРґр°ё
МмМмПтЙПТ»±ѕєГѕнПµБРґр°ё РЎС§Йъ10·ЦЦУУ¦УГМвПµБРґр°ё
РЎС§Йъ10·ЦЦУУ¦УГМвПµБРґр°ё
| Дкј¶ | ёЯЦРїОіМ | Дкј¶ | іхЦРїОіМ |
| ёЯТ» | ёЯТ»Гв·СїОіМНЖјцЈЎ | іхТ» | іхТ»Гв·СїОіМНЖјцЈЎ |
| ёЯ¶ю | ёЯ¶юГв·СїОіМНЖјцЈЎ | іх¶ю | іх¶юГв·СїОіМНЖјцЈЎ |
| ёЯИэ | ёЯИэГв·СїОіМНЖјцЈЎ | іхИэ | іхИэГв·СїОіМНЖјцЈЎ |
їЖДїЈєёЯЦР»ЇС§ АґФґЈєІ»Пк МвРНЈєКµСйМв

Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈєІ»Пк МвРНЈєМоїХМв
| AЈ®СОЛб | BЈ®NaOHИЬТє | CЈ®CuO | DЈ®±ҐєНµДNaHSO3ИЬТє |
| | »мєПОп | ЛщУГКФјБ | ЦчТЄІЩЧч |
| ЈЁ1Ј© | BaSO4(I2) | | |
| ЈЁ2Ј© | CaCO3(SiO2) | | |
| ЈЁ3Ј© | SO2(HCl) | | |
| ЈЁ4Ј© | CO2(CO) | | |
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈєІ»Пк МвРНЈєМоїХМв

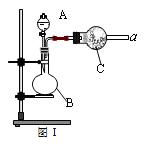
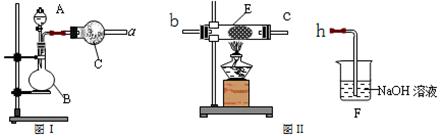
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈєІ»Пк МвРНЈєµҐСЎМв
| AЈ®ОЮЛ®ВИ»ЇёЖ | BЈ®ЅрКфДЖ | CЈ®ОЮЛ®БтЛбН | DЈ®ЙъКЇ»Т |
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈєІ»Пк МвРНЈєµҐСЎМв
| AЈ®ТєдеєНЛДВИ»ЇМј | BЈ®ТТґјєНТТЛб |
| CЈ®ВИ»ЇёЖєНМјЛбДЖИЬТє | DЈ®±ЅєНХфБуЛ® |
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈєІ»Пк МвРНЈєµҐСЎМв
| ІЅЦи | ІЩ Чч | ПЦ Пу |
| ЈЁ1Ј© | ИЎЙЩБїИЬТєµОјУјёµОКЇИпКФТє | ИЬТє±дА¶ |
| ЈЁ2Ј© | БнИЎЙЩБїИЬТєµОјУ№эБїВИЛ®Ј¬ФЩјУИлCCl4ХсµґЈ¬ѕІЦГ | ЙПІгОЮЙ«,ПВІгіКіИємЙ« |
| ЈЁ3Ј© | ИЎ(2)ЙПІгИЬТє,јУИл№эБїBa(NO3)2ИЬТєєНПЎHNO3Ј¬ №эВЛ №эВЛ | УР°ЧЙ«іБµнІъЙъ |
| ЈЁ4Ј© | ПтЈЁ3Ј©µДВЛТєЦРјУИл№эБїAgNO3ИЬТєєНПЎHNO3 | УР°ЧЙ«іБµнІъЙъ |
| AЈ®їЙДЬє¬УР ClТ»Ўў SO32Т»ЎўSO42Т» | BЈ®їП¶ЁГ»УР Ba2 +ЎўClТ»ЎўBrТ» |
| CЈ®І»ДЬИ·¶Ё Na+ Ўў SO32Т»ЎўSO42Т» | DЈ®їП¶Ёє¬УР Na+ЎўBrТ»ЎўSO32Т» |
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈєІ»Пк МвРНЈєµҐСЎМв
| AЈ®H2OЎўNaHCO3ИЬТєЎўСОЛбЎў°±Л®ЎўПхЛб |
| BЈ®°±Л®ЎўNa2CO3ИЬТєЎўПЎБтЛбЎўПЎПхЛбЎўKCNИЬТє |
| CЈ®NaHCO3ИЬТєЎўПЎБтЛбЎўKCNИЬТєЎўNaOHИЬТєЎў°±Л® |
| DЈ®H2OЎўNaOHИЬТєЎўСОЛбЎўПхЛбЎўKCNИЬТє |
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈєІ»Пк МвРНЈєµҐСЎМв
| AЈ®јмСйNa2O2КФСщКЗ·с±дЦКОЄNa2CO3Ј¬ПтКФСщЦРјУИлСОЛбЈ¬ІъЙъОЮЙ«ОЮО¶µДЖшМе |
| BЈ®іэИҐґЦСОЦРµДCa2+ЎўSO42-ЈєТАґОјУИл№эБїBaCl2ИЬТєЎўNa2CO3ИЬТєЈ¬№эВЛєуФЩјУККБїСОЛб |
| CЈ®јмСйХбМЗЛ®ЅвІъОпКЗ·сѕЯУР»№ФРФЈєПтЛ®ЅвєуµДИЬТєЦРјУИлРВЦЖCuЈЁOHЈ©2ІўјУИИ |
| DЈ®јмСйИЬТєЦРКЗ·сТ»¶Ёє¬УРCO2-3ЈєµОјУПЎСОЛбЈ¬Ѕ«ІъЙъµДЖшМеНЁИліОЗеКЇ»ТЛ® |
Ійїґґр°ёєНЅвОц>>
№ъјКѧУУЕСЎ - Б·П°ІбБР±н - КФМвБР±н
єю±±КЎ»ҐБЄНшОҐ·ЁєНІ»БјРЕПўѕЩ±ЁЖЅМЁ | НшЙПУРє¦РЕПўѕЩ±ЁЧЁЗш | µзРЕХ©ЖѕЩ±ЁЧЁЗш | ЙжАъК·РйОЮЦчТеУРє¦РЕПўѕЩ±ЁЧЁЗш | ЙжЖуЗЦИЁѕЩ±ЁЧЁЗш
ОҐ·ЁєНІ»БјРЕПўѕЩ±Ёµз»°Јє027-86699610 ѕЩ±ЁУКПдЈє58377363@163.com