
��08��ɳ������ѧһģ������˵���д�����ǣ� ��
��������������ˮ�����绯���ۻ��ȶ��ǻ�ѧ�仯
�ڸ������������������������仯
�۸��Ͳ����ͣ�����Ǽ��ȩ��֬���������ɱ����DZ�
���л�ѧ�����ѵı仯�ǻ�ѧ�仯
��0.05 mol���ڵ�KHSO4�к��������ӵ���ĿΪ0.1 NA��NA��ʾ�����ӵ�������
����������һ����������������
���ɲ�ͬ��ԭ�ӹ��ɵĴ�����һ���ǻ�����
������뻯ѧ�ܵ��ת��������һ���������ʲ���
����Ӿ���Ļ�ѧ��Խǿ�����ۡ��е��Խ��
A���٢ۢܢݢ� B���٢ڢܢޢ��
C���ڢܢݢޢߢ� D���ڢۢݢߢ�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��08��ɳ������ѧһģ����16�֣��������е�A��B��C��D��E 5��Ԫ�أ�ԭ������������A��Dͬ���壻A��B��ԭ������֮�͵���C��ԭ��������C2��������D+���ӵĺ����������ȣ�Bԭ����D+���Ӻ��������֮�͵���Eԭ�ӵĺ������������D��E��Ԫ�����γ���������Ϊ1��1�����ӻ�����.
��1��C��Ԫ������Ϊ_______��
��2��A��BԪ���γɻ�����B2A4�������ʽΪ ��B2A4һ����ȼ�ϵ����һ�ּ���ȼ�ϵ�أ��������Һ��20��һ30����KOH ��Һ���ŵ�ʱ����B2��ˮ�����ȼ�ϵ�طŵ�ʱ�������ĵ缫��Ӧʽ��_____��
��3����������ʯīΪ�缫��⺬0.2mol DE��ˮ��Һʱ�������ĵ缫��ӦʽΪ__________����������0.2mol����ʱ����ô�ʱ��Һ�����Ϊ200mL,�����Һ��pH��ӽ�����ֵΪ ��
��4��A��B��C��D��E�е���������Ԫ�ؿ���ɶ��ֻ����X��Y�����е����֣�X��Y���ǼȺ����Ӽ��ֺ����ۼ������ӻ������X��Y�ֱ�����ˮʱ��X�ܴٽ�ˮ�ĵ��룬��Y������ˮ�ĵ��룬Xˮ��Һ��pH<7��Yˮ��Һ��pH>7.��0.2mol?L��1��X��Һ��0.1mol?L��1��Y��Һ�������Ϻ���Һ�ʼ���.���жϣ�
��X�Ļ�ѧʽΪ__________��Y�Ļ�ѧʽΪ_________ ��
�ڻ����Һ�и��ִ������ӵ����ʵ���Ũ���ɴ�С��˳��Ϊ_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��08��ɳ������ѧһģ����12�֣���A��B��C��D��E���ֳ���������������±��е������γɵģ�
������ | K+ Ba2+ Cu2+ Al3+ |
������ | SO42- HCO3�� NO3�� OH�� |
Ϊ�˼�������������ֱ��������ʵ�飬�����ǣ�
�ٽ���������ˮ��DΪ��ɫ��Һ��������Ϊ��ɫ��Һ��
�ڽ�E��Һ��ε��뵽C��Һ�г��ְ�ɫ�����������μ������������������������ӵ������������ʵ���ȴ���٣�
�۽�����ɫ��Ӧ��A��B��CΪ��ɫ������ɫ�ܲ�������
�ܸ���Һ�м������ᱵ��Һ���ټӹ���ϡ���ᣬֻ��A�зų���ɫ���壬C��D�в�����ɫ������
�ݽ�B��D����Һ��ϣ�δ���������������ɣ�
�������ʵ����գ�
��д��B��D�Ļ�ѧʽ��B ��D ��
�ƽ���1 mol A����Һ�뺬1 mol E����Һ��������ӷ���ʽΪ ��
�ǽ�E��Һ��ε��뵽��һ����C����Һ�г��������ﵽ���ֵʱ�����ӷ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��08��ɳ������ѧһģ����16�֣�ij����ʵ��С��ѧ��������ͼ��ʾװ�ý��С��Ҷ��ᣨ�������ᣩ�������ȷֽ⡱��ʵ�飬����֤�ֽ��������CO2��CO����ͼ�мг�װ������ȥ��
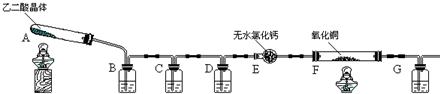 |
��1��װ��C��ʢ�� ��Һ��D�������� ��
��2��֤���ֽ��������CO�������� ��
��3����װ����һ������֮������д�� ��
��4��ijͬѧ����������ϣ������Ƕ�Ԫ�ᣬ���Ա�̼��ǿ�������ȷֽ����������������������ƺͲ�����ƾ�Ϊ��ɫ�������˸�ͬѧ��Ϊ����Ҫ��װ��B֮ǰ����һ��װ��________����д���б����ĸ����ϴ��װ�á�
a��ˮ b��NaOH ��Һ c��Na2CO3 ��Һ d������NaHCO3 ��Һ
��5��������������������±�KMnO4��������Ӧ�����ӷ���ʽΪ��
2MnO4�� + 5H2C2O4 +6 H+ = 2Mn2+ +10 CO2��+8 H2O
ʵ���ҳ��ò����Ʊ궨KMnO4��Һ���������£�ȷ��ȡ2.680 g�����ƣ�������ƿ�У���100 mLϡ�����ܽ⣬����ƿ���� �����ʽ�� ����ʽ�����ζ����£���KMnO4��Һ�ζ����� ʱ�����ﵽ�ζ��յ㡣�ظ������ζ��������Σ�ʵ���������±���ʾ��
| �ζ�ǰ | ��һ���յ� | �ڶ����յ� | �������յ� | ���Ĵ��յ� |
�ζ��� Һ��̶� | 0.00 mL | 20.02 mL | 21.00mL | 19.98 mL | 20.00mL |
KMnO4��Һ�����ʵ���Ũ�ȵ���c(KMnO4)= mol?L��1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��08��ɳ������ѧһģ�����������£���ƿ��������ԭ����һ����ȵ��ǣ� ��
A��ͬ������ͬ�����C2H4��C3H6 B��ͬ�¶ȡ�ͬ�����N2��H2
C��ͬ�����ͬ�ܶ�N2��NO D��ͬ�����ͬѹǿ��N2O��CO2
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com