
��10�֣�����ʵ�顱����װ�ü����������ŵ㣬���ͼʾ�ش��й�����
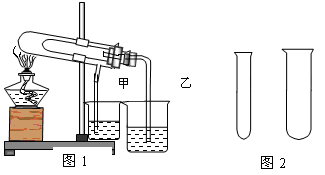
��1��������ͼ1�Ƚ�̼�����ƺ�̼���Ƶ����ȶ��ԣ�������ֽ������Ӧ�ڴ��Թܵײ����� ���ѧʽ����ʯ��ˮ����ǵ��ձ��� (��ס���) ��
��д���Թ��з�Ӧ�Ļ�ѧ��Ӧ����ʽ��
��2��ʹ��ͼ2�д�С��һ���Թܣ������ۺ�����������Һ��ȡ���������ռ�һ�Թ�������
�����ۺ�����������Һ���� �Թ���(���С)��ɷ���װ�ã���д���÷�Ӧ������
����ʽΪ�� Ϊ��ʹ�����ռ��������ܽ�
�����س������ռ��������Թܣ�Ӧ��β�����д�����µIJ��裺
��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��10�֣�����ʵ�顱����װ�ü����������ŵ㣬���ͼʾ�ش��й�����
��1��������ͼ1�Ƚ�̼�����ƺ�̼���Ƶ����ȶ��ԣ�������ֽ������Ӧ�ڴ��Թܵײ����� ���ѧʽ����ʯ��ˮ����ǵ��ձ��� (��ס���) ��
��д���Թ��з�Ӧ�Ļ�ѧ��Ӧ����ʽ��
��2��ʹ��ͼ2�д�С��һ���Թܣ������ۺ�����������Һ��ȡ���������ռ�һ�Թ�������
�����ۺ�����������Һ���� �Թ���(���С)��ɷ���װ�ã���д���÷�Ӧ������
����ʽΪ�� Ϊ��ʹ�����ռ��������ܽ�
�����س������ռ��������Թܣ�Ӧ��β�����д�����µIJ��裺
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ����ѧ����ѧ��һ��ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ�ʵ����
��10�֣�����ʵ�顱����װ�ü����������ŵ㣬���ͼʾ�ش��й�����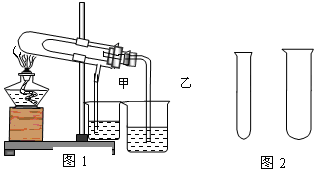
��1��������ͼ1�Ƚ�̼�����ƺ�̼���Ƶ����ȶ��ԣ�������ֽ������Ӧ�ڴ��Թܵײ����� ���ѧʽ����ʯ��ˮ����ǵ��ձ��� (��ס���) ��
��д���Թ��з�Ӧ�Ļ�ѧ��Ӧ����ʽ��
��2��ʹ��ͼ2�д�С��һ���Թܣ������ۺ�����������Һ��ȡ���������ռ�һ�Թ�������
�����ۺ�����������Һ���� �Թ���(���С)��ɷ���װ�ã���д���÷�Ӧ������
����ʽΪ�� Ϊ��ʹ�����ռ��������ܽ�
�����س������ռ��������Թܣ�Ӧ��β�����д�����µIJ��裺
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��0111 ��ĩ�� ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ����ĩ�� ���ͣ�ʵ����
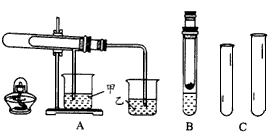
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com