
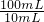 =0.01mol��
=0.01mol��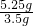 =0.015mol������ˮ�����ʵ���Ϊ
=0.015mol������ˮ�����ʵ���Ϊ =0.12mol������1��n=0.015mol��0.12mol�����n=8��
=0.12mol������1��n=0.015mol��0.12mol�����n=8��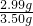 ��100%=85.4%��
��100%=85.4%��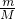 ����ᾧˮ�����ʵ��������ݣ�1��3.50g��������Ba��OH��2�����ʵ�������5.25g��������Ba��OH��2�����ʵ���������Ba��OH��2�����ʵ�����ᾧˮ�����ʵ�����ϵ����n��ֵ��
����ᾧˮ�����ʵ��������ݣ�1��3.50g��������Ba��OH��2�����ʵ�������5.25g��������Ba��OH��2�����ʵ���������Ba��OH��2�����ʵ�����ᾧˮ�����ʵ�����ϵ����n��ֵ��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| �ζ� ���� |
������Һ����� /mL |
����Һ�����/mL | �ζ�ǰ�̶� | �ζ���̶� | 1 | 10.00 | 1.02 | 21.03 | 2 | 10.00 | 2.00 | 21.99 | 3 | 10.00 | 0.20 | 20.20 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������һ��ʹ�ù㷺�Ļ�ѧ�Լ���ij����С��ͨ������ʵ��ⶨij������Ba(OH)2��nH2O�ĺ�����
��1����ȡ3.50 g������������ˮ���100 mL��Һ������ȡ��10.0 mL��Һ����ƿ�У���0.100 mol / L HCl����Һ��Ӧ����ȫ��Ӧ�����ı�Һ20.0 mL�����ʲ����ᷴӦ�������������������������ʵ�����
��2����ȡ5.25 g����������ʧȥȫ���ᾧˮ�����ʲ��ֽ⣩���Ƶ�����Ϊ3.09 g����Ba(OH)2��nH2O�е�nֵ��
��3��������Ba(OH)2��nH2O����������Ϊ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��6�֣�����������һ��ʹ�ù㷺�Ļ�ѧ�Լ���ij����С��ͨ������ʵ��ⶨij������Ba(OH)2��nH2O�ĺ�����
��1����ȡ3.50g������������ˮ���100mL��Һ������ȡ��10.0mL��Һ����ƿ�У���0.100mol/LHCl��Һ�кͣ�����������20.0mL�����ʲ����ᷴӦ�������������������������ʵ�����
��2����ȡ5.25g����������ʧȥȫ���ᾧˮ�����ʲ��ֽ⣩���Ƶ�����Ϊ3.09g����Ba(OH)2��nH2O�е�nֵ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com