
��20�֣�ʵ����������0.1 mol / L��NaOH��Һ350 mL���ش��������⣺
��1������ʱ����ʹ�õ���������ƽ�������룩���ձ��������� ��
��2�������������� ��
��3����������ƽ��������ҪNaOH������Ϊ g��
��4������ʱ��һ��ɷ�Ϊ���¼������裺�ٳ��� �ڼ��� ���ܽ� ��ҡ�� ��ת�� ��ϴ��
�߶��� ����ȴ ��װƿ��ǩ������ȷ�IJ���˳��Ϊ ��
��5�����ݹ���������С�ij����̶��ߣ����� ��
��6�����в����лᵼ����Һʵ��Ũ�ȳ�����������á�ƫ�ߡ�����ƫ�͡����͡���Ӱ�족�ش�
a ����ʱ����� ��
b ����ʱ���ӿ̶��� ��
c ��Һǰ����ƿ��������ˮ ��
d ����ʱ��ˮ�Ӷ��ˣ����õι����� ��
e δϴ�Ӳ��������ձ� ��
��1��500ml����ƿ����ͷ�ιܣ���2�����衢��������3��2.0 g����4���ڢ٢ۢ�ݢޢߢܢ��5���������� ����6�� aƫ�ͣ�bƫ�ߣ�c��Ӱ�죻dƫ�ͣ�eƫ�͡�
��������
�������������ʵ��������350mL����ƿ�����ݡ����������ԭ��Ӧѡȡ500mL����ƿ������Һ�����ƣ�
��1������һ�����ʵ���Ũ����Һ�õ�������������ƽ����Ͳ��500mL����ƿ���ձ�������������ͷ�ιܡ�ҩ�ȣ���2�������ƹ����У���Ҫ�����������ձ��е���Һ���ٽ�������ܽ⣬��Ҫ�����������������ã��Է�ֹ��Һ��������3��0.1mol/L��NaOH��Һ500mL����n=0.5L��0��1mol=0.05mol��NaOH��Ħ������Ϊ40g/mol������ҪNaOH������Ϊ��m=n��M=0.05mol��40g/mol=2.0g����4�����������г������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣬��ȴ��ת�Ƶ�500mL����ƿ�У����ò�����������Ȼ��ϴ���ձ��Ͳ���������ϴ��Һת������ƿ������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ����װ���Լ�ƿ�У����Բ���˳��Ϊ�ڢ٢ۢ�ݢޢݢߢܢ��5������ƿΪ����������һ�����ƹ���������Һ�������ˮ�����̶��ߣ���ȷ�ķ��������������ƣ���6��a������ֽ����NaOH�����������������տ�����ˮ�Ͷ�����̼����ȡ���������Ƶ��������٣�nƫС����Ũ��ƫС��b������ʱ����������ƿ�̶��ߣ�ʹ��Һ�����ƫ�ͣ�������ҺŨ��ƫ�ߣ�c�������Ҫ���ݣ�����ƿ�����������������ˮ������ҺŨ����Ӱ�죻d�������̶��ߣ���Һ�������ʹ����һ����ˮ����ҺҲ�Ѿ������꣬��ҺŨ��ƫ�ͣ�e��δϴ���ձ�����������������������մ���ձ����벣�����ϣ��������Ƶ�ʵ��������С����ҺŨ��ƫ�͡�
���㣺����һ�����ʵ���Ũ����Һ�����ơ�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2014��㶫ʡ�����и߶���ѧ�����п��Ի�ѧ���ģ��Ծ��������棩 ���ͣ�ѡ����
�����ڸ߷��ӻ��������
A������ B����ά�� C�������� D����֬
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��㶫ʡ�����и߶���ѧ�����п��Ի�ѧ���ģ��Ծ��������棩 ���ͣ�ѡ����
�������ʲ�����ΪʳƷ���Ӽ�����
A���״� B��ʳ�� C�������� D����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�ﰲ��ʡ�߶���ѧ�����л�ѧ�Ծ��������棩 ���ͣ�ѡ����
������ѧ��Ӧ���ʵ�����Ҫ������
A���¶Ⱥ�ѹǿ B����Ӧ���Ũ��
C���μӷ�Ӧ�ĸ����ʵ����� D�������ļ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�ﰲ��ʡ�߶���ѧ�����л�ѧ�Ծ��������棩 ���ͣ�ѡ����
ͭпԭ��أ���ͼ��ʾ�������类�о��ĵ�ء����ڸõ�ص�˵������ȷ����
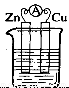
A��ͭƬ�Ǹ���
B���������
C�����Ӵ�ͭƬ����������пƬ
D��ͭƬ����û������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�ﰲ��ʡ��һ��ѧ�����л�ѧ�Ծ��������棩 ���ͣ�ѡ����
������Һ�У� n��Na+��������
A.4L 0.5 mol/L NaCl ��Һ
B.1L 0.3 mol/L Na2SO4��Һ
C.0.8L 0.4 mol/L NaOH ��Һ
D.2L 0.15 mol/L Na3PO4��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�ﰲ��ʡ��һ��ѧ�����л�ѧ�Ծ��������棩 ���ͣ�ѡ����
��һС����Ͷ��ʢ��5mL���ͳ���ʯ��ˮ���Թ�������ܹ۲쵽��������
A�����۳�С����Һ�����ζ� B������������
C����Һ�ײ�������ɫ�������� D����Һ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�ﰲ��ʡ�����и߶���ѧ�����л�ѧ�������Ծ��������棩 ���ͣ�ѡ����
������ͼװ�õ�˵����ȷ����
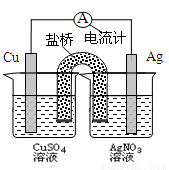
A�����缫�Ǹ���
B��ͭ�缫�Ϸ����ķ�ӦΪCu-2e��=Cu2��
C�����·�еĵ����Ǵ����缫����ͭ�缫��
D����װ���ܽ�����ת��Ϊ��ѧ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�^����ʡ��һ��ѧ�ڵ�һ���ʼ컯ѧ�Ծ��������棩 ���ͣ������
��10�֣���һ�������������Na2CO3��Na2SO4��CuSO4��NaCl�Ȼ����ɣ�Ϊ�˼����������������ʣ���������ʵ�顣
�ٽ���������ˮ�������õ���ɫ����Һ��
��������Һ�еμ����ᱵ��Һ���а�ɫ�������ɣ�
�۹��ˣ�����������ϡ�����У����ֳ���ȫ���ܽ⡣
��1�����жϣ����������п϶�����_________���϶�û��__________�����ܺ���__________��
��2���Կ����е����ʣ��ɲ�������Һ�еμ�___________________�����Լ����ƣ������飬������и����ʣ���������____________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com