�⣺��1����������Cu��H
2SO
4��������100%��������Ⱦ����������Cu��������100%��H
2SO
4��������50%���Ҳ�����Ⱦ��ʴ𰸣�ѡ����ԭ�������ʸߣ���Ⱦ�٣�
��2������Һ������������������ͭ��Ӧ��������ͭ��2Cu+O
2
2CuO������ͭ�������ᷴӦ��CuO+H
2SO
4=CuSO
4+H
2O����ʽ��ӣ�2Cu+O
2+2H
2SO
4
2CuSO
4+2H
2O�������ʱ���뼸��
H
2O
2����ҺҲ��ܿ����ɫ��˵��H
2O
2�ܽ�ͭ������ͭ���ӣ����ӷ�ӦΪCu+H
2O
2+2H
+
Cu
2++2H
2O���ʴ𰸣�2Cu+O
2+2H
2SO
4
2CuSO
4+2H
2O��Cu+H
2O
2+2H
+
Cu
2++2H
2O��
��3����֪��2Cu��s��+

O
2��g��=Cu
2O��s������H
1=-12.0kJ/mol ��
2Cu��s��+S��s��=Cu
2S��s������H
2=-79.5kJ/mol ��
S��s��+O
2 ��g��=SO
2��g������H
3=-296.83kJ/mol ��
���ݸ�˹���ɢ�-�١�2-�ڿɵ�Cu
2S��s��+2Cu
2O��s��=6Cu��s��+SO
2��g������H
4=-193.33kJ/mol��
�ʴ�Ϊ��-193.33��
��4�����ݻ��ϼ�����������ȿ�֪��
Cu
2S Cu��+1��+2��2 ����10��3
S��-2��+6��8
HNO
3 N��+5��+2��3��10
����ƽ��Ļ�ѧ��ӦΪ3Cu
2S+16HNO
3
3CuSO
4+3Cu��NO
3��
2+10NO��+8H
2O��
�ʴ𰸣�3��16��3��3��10��8H
2O��
��5����Cu
2S ������Ϊx��CuS ������Ϊy��
Cu
2S��2CuO CuS��CuO
160 160 96 80
x x y
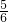
y
���������飺

��ã�x=6m
2-5m
1��y=6m
1-6m
2��
�ʴ�Ϊ��6m
2-5m
1��
��������1������ԭ�������ʺͻ����ĽǶȣ�
��2��������Һ���������μӷ�Ӧ��H
2O
2����������������
��3�����ݸ�˹���ɼ��㷴Ӧ�ȣ�
��4������������ԭ��Ӧ�л��ϼ�����������ȼ������غ�����ƽ��
��5������Cu
2S��CuS���Ⱥ����ն����CuO��������������⣮
������������Ҫ������ͭ�Ļ�ѧ���ʣ�ͬʱҲ�����˸�˹���ɵ����ã�������ԭ��Ӧ��������������ۺ��ԣ�

 2CuO���������� CuO+H2SO4=CuSO4+H2O������ ��Cu+2H2SO4��Ũ��
2CuO���������� CuO+H2SO4=CuSO4+H2O������ ��Cu+2H2SO4��Ũ�� CuSO4+SO2��+2H2O
CuSO4+SO2��+2H2O O2��g��=Cu2O��s������H1=-12.0kJ/mol
O2��g��=Cu2O��s������H1=-12.0kJ/mol ��CuSO4+��Cu��NO3��2+��NO��+��______
��CuSO4+��Cu��NO3��2+��NO��+��______ 2CuO������ͭ�������ᷴӦ��CuO+H2SO4=CuSO4+H2O����ʽ��ӣ�2Cu+O2+2H2SO4
2CuO������ͭ�������ᷴӦ��CuO+H2SO4=CuSO4+H2O����ʽ��ӣ�2Cu+O2+2H2SO4 2CuSO4+2H2O�������ʱ���뼸��
2CuSO4+2H2O�������ʱ���뼸�� Cu2++2H2O���ʴ𰸣�2Cu+O2+2H2SO4
Cu2++2H2O���ʴ𰸣�2Cu+O2+2H2SO4 2CuSO4+2H2O��Cu+H2O2+2H+
2CuSO4+2H2O��Cu+H2O2+2H+ Cu2++2H2O��
Cu2++2H2O��  O2��g��=Cu2O��s������H1=-12.0kJ/mol ��
O2��g��=Cu2O��s������H1=-12.0kJ/mol �� 3CuSO4+3Cu��NO3��2+10NO��+8H2O��
3CuSO4+3Cu��NO3��2+10NO��+8H2O��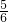 y
y 



 2CuO CuO+H2SO4=CuSO4+H2O ��Cu+2H2SO4��Ũ��
2CuO CuO+H2SO4=CuSO4+H2O ��Cu+2H2SO4��Ũ�� CuSO4+SO2��+2H2O
CuSO4+SO2��+2H2O 2CuO CuO+H2SO4=CuSO4+H2O ��Cu+2H2SO4��Ũ��
2CuO CuO+H2SO4=CuSO4+H2O ��Cu+2H2SO4��Ũ�� CuSO4+SO2��+2H2O
CuSO4+SO2��+2H2O