
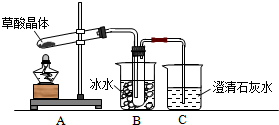
���� ��1�����ᾧ�壨H2C2O4•2H2O����ɫ���۵�Ϊ101�棬������ˮ��������ˮ��������170�����Ϸֽ⣬����������ȷֽ⣬�ֽ�ʱ�����������̼��ʹ����ʯ��ˮ����ǣ���������κͼ���������ˮ���������������ˮ��Bװ���¶Ƚϵͣ����������ã���ֹ����ʵ�飻
��2����Ҫ��������CO���ڼ���ʵ�����Ũ�������Ƴ�ȥ������̼���ó���ʯ��ˮ���������̼���ü�ʯ�Ҹ���CO������CO�Ļ�ԭ�Խ�CO�����������ó���ʯ��ˮ�������ɵĶ�����̼������ˮ���ռ�CO��Hװ����ʢ�ŵ�����Ӧ�þ��������ԣ��Һ�CO��Ӧ��������������
��CO���л�ԭ�ԣ������������Ƕ�����̼��������̼��ʹ����ʯ��ˮ����ǣ�
��3����Ҫ֤���������Դ���̼�ᣬ��������ǿ����ȡ���
����������к͵ζ�������ʵ�����ȷ�������Ƕ�Ԫ�
��4����KMnO4�Ͳ�������ϡ�����з�Ӧ��������ء������̡�������̼��ˮ��
�ڼ���KMnO4��Һ��Ũ��Ϊc�����ݷ���ʽ2MnO4-+5C2O42-+16H+=2Mn2++10CO2��+8H2O���㣮
��� �⣺��1�����ᾧ�壨H2C2O4•2H2O����ɫ���۵�Ϊ101�棬������ˮ��������ˮ��������170�����Ϸֽ⣬����������ȷֽ⣬�ֽ�ʱ�����������̼��������̼���������Ʒ�Ӧ���������Ե�̼��Ƴ�����ʹ����ʯ��ˮ����ǣ�����C�й۲쵽�������ǣ�������ð���ҳ���ʯ��ˮ����ǣ�˵���ж�����̼���ɣ�
��������κͼ���������ˮ���������������ˮ�������ӷ����������ɵ������к��в��ᣬ������������Ʒ�Ӧ���������ԵIJ���ƶ����Ŷ�����̼�ļ��飬Bװ���¶Ƚϵͣ����������ã���ֹ���Ŷ�����̼�ļ��飬
�ʴ�Ϊ��������ð��������ʯ��ˮ����ǣ�CO2��������ˮ�����Ͳ��ᣩ����ֹ�������װ��C��Ӧ���ɳ���������CO2�ļ��飻
��2����Ҫ��������CO���ڼ���ʵ�����Ũ�������Ƴ�ȥ������̼���ó���ʯ��ˮ���������̼���ü�ʯ�Ҹ���CO������CO��CuO������ԭ��Ӧ����CO2�������ó���ʯ��ˮ�������ɵĶ�����̼������ˮ���ռ�CO�������Ⱦ������������˳����A��B��F��D��G��H��D��I��
Hװ����ʢ�ŵ�����Ӧ�þ��������ԣ��Һ�CO��Ӧ��������������CuO�ܱ�CO��ԭ�ҷ�Ӧ�����к�ɫ�����Ϊ��ɫ���������ԣ�����H��ʢ�ŵ�������CuO��
�ʴ�Ϊ��F��D��G��H��D��I��CuO��
��CO���л�ԭ�ԣ������������Ƕ�����̼��������̼��ʹ����ʯ��ˮ����ǣ���CO����ɫ��CuO��ԭΪ��ɫ��Cu��ֻҪH�к�ɫ����ת��Ϊ��ɫ������Dװ����Һ����Ǿ�˵������CO��
�ʴ�Ϊ��H�к�ɫ��ĩ��Ϊ��ɫ������D�г���ʯ��ˮ����ǣ�
��3����Ҫ֤���������Դ���̼�ᣬ��������ǿ����ȡ���ᣬ��ʢ������NaHCO3���Թ���μӲ�����Һ�������ݲ�����˵���������Դ���̼�ᣬ
�ʴ�Ϊ����ʢ������NaHCO3���Թ���μӲ�����Һ�������ݲ�����˵���������Դ���̼�
�ڲ����NaOH�����кͷ�Ӧʱ����������Ƕ�Ԫ�ᣬ��μӷ�Ӧ�IJ������ʵ���Ӧ����NaOH��һ�룬������NaOH����Һ�ζ�������Һ������NaOH�����ʵ����Dz����2����˵�������Ƕ�Ԫ�ᣬ
�ʴ�Ϊ����NaOH����Һ�ζ�������Һ������NaOH�����ʵ����Dz����2����
��4����KMnO4�Ͳ�������ϡ�����з�Ӧ��������ء������ơ������̡�������̼��ˮ����Ӧ�����ӷ���ʽΪ��2MnO4-+5C2O42-+16H+=2Mn2++10CO2��+8H2O��
�ʴ�Ϊ��2MnO4-+5C2O42-+16H+=2Mn2++10CO2��+8H2O��
�ڸ��������������ݼ�����������Һ���ƽ��ֵΪ=$\frac{16.02+16.0+16.01}{3}$=16.01mL������KMnO4��Һ��Ũ��Ϊc��n��C2O42-��=$\frac{0.2000g}{134g/mol}$��
��2MnO4-+16H++5C2O42-=2Mn2++10CO2��+8H2O
2mol 5mol
c��0.01601L $\frac{0.2000g}{134g/mol}$
���c=$\frac{0.2000g��2mol}{5mol��134g/mol��0.01601L}$��
�ʴ�Ϊ��$\frac{0.2000g��2mol}{5mol��134g/mol��0.01601L}$��
���� �������Ҷ���ķֽ�Ϊ����������ʵ�鷽����ƣ�Ϊ��Ƶ���㣬���ؿ���ѧ��֪ʶ�ۺ�Ӧ�á�ʵ���������������ʵ�鷽������������ۺ��Խ�ǿ���ѵ�������ʵ���Ⱥ�˳����ʵ��Ŀ�ļ����ʵ����ʽ�������˳��ע��Ҫ�ų��������ظ��ţ���Ŀ�Ѷ��еȣ�


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
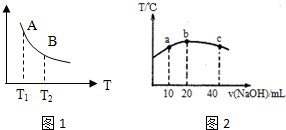
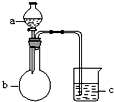 ����ͼ��ʾװ�ý�������ʵ�飬a��b��c����װ�Լ����±���ʾ������ʵ����������۶�Ӧ��ϵ����ȷ����һ���ǣ�������
����ͼ��ʾװ�ý�������ʵ�飬a��b��c����װ�Լ����±���ʾ������ʵ����������۶�Ӧ��ϵ����ȷ����һ���ǣ�������| ѡ�� | a | b | c | ���� | ���� |
| A | ����ʳ��ˮ | ̼���� | ����KMnO4��Һ | c����Һ��ɫ��ȥ | ��Ȳ���л�ԭ�� |
| B | Ũ���� | KMnO4���� | NaBr��Һ | c����Һ����ɫ���ɫ | Cl2�������Ա�Br2ǿ |
| C | ϡ���� | ����ʯ | Na2SiO3��Һ | c���а�ɫ��״�������� | ̼������Աȹ���ǿ |
| D | ���� | Na2SO3���� | Ʒ����Һ | c����Һ��ɫ��ȥ | SO2����Ư���� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
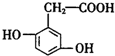 ��������˵��������ǣ�������
��������˵��������ǣ�������| A�� | 1mol�������������Ũ��ˮ��Ӧ���������3mol Br2 | |
| B�� | 1mol������������4mol H2��Ӧ | |
| C�� | ������������ͬһƽ���ϵ�̼ԭ��������7�� | |
| D�� | ��������̼��������Һ��Ӧ�ų�CO2������2.24LCO2������£���Ҫ�����16.8g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ��ѧʽ | ���볣�� |
| CH3COOH | Ka=1.76��10-5 |
| H2SO3 | ${K_{a_1}}$=1.54��10-2 |
| ${K_{a_2}}$=1.02��10-7 | |
| HF | Ka=6.03��10-4 |
| A�� | 1mol•L-1NaHA��Һ��һ�����ڣ�c��Na+��=c��H2A��+c��HA-��+c��A2-�� | |
| B�� | ���������Һ�м����������ᣬ�õ������Ի����Һ�У�c��Na+����c��CH3COO-����c��H+����c��OH-�� | |
| C�� | pH������3�Ĵ�����������Һ�������Ϻ���Һ��pH��� | |
| D�� | ��֪ij�¶��³�������ĵ���ƽ�ⳣ���������ͬ���ʵ���Ũ�ȵ�CH3COONa��NaF��Na2SO3��NaHSO3ˮ��Һ����Һ������������С�������е�˳����Na2SO3��CH3COONa��NaF��NaHSO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �Ҷ����ͱ�������Ϊͬϵ�� | |
| B�� | ��ͬԪ�ص�ԭ�ӹ��ɵķ���ֻ�����Թ��ۼ� | |
| C�� | ${\;}_{92}^{235}$U��${\;}_{92}^{238}$U����������ͬ��������ͬ��ͬ�ֺ��� | |
| D�� | �����ڵڢ�A���A��Ԫ�ص�ԭ�Ӽ乹�ɵķ��ӣ�������ԭ�������8���ӽṹ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
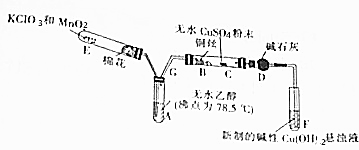
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ԭ�Ӱ뾶A��B��D��C | B�� | ԭ������a��b��c��d | ||
| C�� | ���Ӱ뾶D��C��B��A | D�� | ������B��A���ǽ�����D��C |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com