
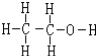 ��
��| ʵ����� | 1 | 2 | 3 |
| Na2S2O3��Һ�����mL�� | 19.98 | 20.02 | 21.18 |
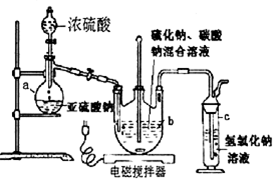
���� ��1������װ��ͼ������
��2��Na2S2O3�����������»�����S�Ͷ�������
��3��������Ϣ��֪��������ƾ�������ֽ⣬�����ɻ�ʹ�����������ˮ���ֽ⣻�γɾ�Ĥ����Ϊ�������壬������������Ϊ�¶Ƚϵͣ�
��4�����������������ˮ���������Ҵ��������Ҵ���ϴ�ӣ�
��5�����ݷ���ʽ�ҳ���ϵʽ��IO3-��6S2O32-�����ݵ�������ӵ����ʵ��������������Ƶ����ʵ���Ũ�ȣ����250mL��Һ����������ƾ������������������5g��ȵô��ȣ�
A���ζ���ĩ��Na2S2O3��Һ��ϴ����Na2S2O3��Һ�ᱻϡ�ͣ�
B���ζ��յ�ʱ���Ӷ�����ʹNa2S2O3��Һ���ƫ��
C����ƿ������ˮ��ϴ����ʵ����ûӰ�죻
D���ζ��ܼ��촦�ζ�ǰ�����ݣ��ζ��յ㷢�����ݣ�ʹ������Na2S2O3�������С��
��� �⣺��1����װ��ͼ��֪aװ��Ϊ������ƿ���ʴ�Ϊ��������ƿ��
��2��Na2S2O3�����������»�����S�Ͷ����������Բ��ʻ��½����䷴Ӧ�����ӷ���ʽΪS2O32-+2H+=S��+H2O+SO2����
�ʴ�Ϊ��S2O32-+2H+=S��+H2O+SO2����
��3����֪��������ƾ�������ֽ⣬�����ɻ�ʹ�����������ˮ���ֽ⣬���Դ������������Һ�з�������ʱ���ܽ���Һ�������ɣ�����Ũ�������������Һ�����¶Ƚϵ�ʱ���������壬����ȴʱ����Һ�����¶Ƚϵͣ����������壬
�ʴ�Ϊ�����ɻ�ʹ�����������ˮ���ֽ⣻��Ϊ��Һ�����¶Ƚϵͣ�
��4�����������������ˮ���������Ҵ�����ϴ����������ƾ���ʱ��Ϊ�˼��پ�����ܽ⣬���Ҵ���ϴ�ӣ��Ҵ��ĽṹʽΪ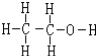 ��
��
�ʴ�Ϊ��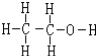 ��
��
��5������ƿ�м���25mL 0.0lmol•L-1KIO3��Һ�������������KI���ữ���������з�Ӧ��5I-+IO3-+6H+�T3I2+3H2O���ټ��뼸�ε�����Һ������������Na2S2O3��Һ�ζ���������Ӧ��I2+2S2O32-�T2I-+S4O62-��
��ɵù�ϵʽ��IO3-��6S2O32-��
1mol 6mol
0.025L��0.0lmol•L-1 n��S2O32-��
��n��S2O32-��=0.0015mol��
������ʵ����������ϴ���ȥ��
����250mL�����������Һ����������Ƶ����ʵ���Ϊ0.0015mol��$\frac{250}{��19.98+20.02����\frac{1}{2}}$=0.01875mol��
����������Ƶ�����Ϊ0.01875mol��248g/mol=4.65g��
��ò�Ʒ�Ĵ�����$\frac{4.65}{5}$��100%=93%��
A���ζ���ĩ��Na2S2O3��Һ��ϴ����Na2S2O3��Һ�ᱻϡ�ͣ����Բ����������Ƶ�����ƫС���ʴ���ƫС����Aѡ��
B���ζ��յ�ʱ���Ӷ�����ʹNa2S2O3��Һ���ƫ���������������Ƶ�����ƫС���ʴ���ƫС����Bѡ��
C����ƿ������ˮ��ϴ����ʵ����ûӰ�죬���Ȳ��䣬��C��ѡ��
D���ζ��ܼ��촦�ζ�ǰ�����ݣ��ζ��յ㷢�����ݣ�ʹ������Na2S2O3�������С�������ԭ��Һ�е���������Ƶ�����ƫ����ƫ��D��ѡ��
�ʴ�Ϊ��93%��AB��
���� ����ͨ����ȡNa2S2O3•5H2O��ʵ������������������Ʊ���������ơ�����ʵ����������ӷ���ʽ����д�����ʴ��ȵļ��㡢�ζ��������ȣ���Ŀ�Ѷ��еȣ���ȷʵ���������Ƽ�������ʵ������ǽ����Ĺؼ��������ֿ�����ѧ���ķ������������������Ӧ����ѧ֪ʶ��������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
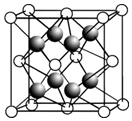 ��1������VSEPRģ���жϣ�������������ԭ�Ӷ���ͬһƽ���ϵ�һ����B��
��1������VSEPRģ���жϣ�������������ԭ�Ӷ���ͬһƽ���ϵ�һ����B��| ������/kJ•mol-1 | I1 | I2 |
| ͭ | 746 | 1958 |
| п | 906 | 1733 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ʵ��� ���� | ������Һ��� V/mL | ����������Һ��� V/mL |
| 1 | 19.90 | 10.00 |
| 2 | 20.10 | 10.00 |
| 3 | 22.00 | 10.00 |
| 4 | 20.00 | 10.00 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �ζ����� | ������Һ �����mL�� | ������� | |
| �ζ�ǰ�Ŀ̶� ��mL�� | �ζ���Ŀ̶� ��mL�� | ||
| ��һ�� | 10.00 | 0.40 | 20.50 |
| �ڶ��� | 10.00 | 4.10 | 24.00 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ʵ�� ��� | KOH��Һ�� Ũ��/mol•L-1 | �ζ����ʱ��KOH��Һ��������/mL | �����ε����/mL�� |
| 1 | 0.10 | 22.62 | 20.00 |
| 2 | 0.10 | 22.72 | 20.00 |
| 3 | 0.10 | 22.80 | 20.00 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
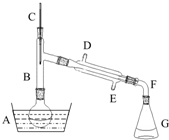 ��������һ����Ҫ���л�����ԭ�ϣ��Ʊ��������ԭ����95%�Ҵ���80%���ᣨ������ˮϡ��Ũ���ᣩ����ϸ���廯�Ʒ�ĩ�ͼ������Ƭ���÷�Ӧ��ԭ�����£�NaBr+H2SO4=NaHSO4+HBr
��������һ����Ҫ���л�����ԭ�ϣ��Ʊ��������ԭ����95%�Ҵ���80%���ᣨ������ˮϡ��Ũ���ᣩ����ϸ���廯�Ʒ�ĩ�ͼ������Ƭ���÷�Ӧ��ԭ�����£�NaBr+H2SO4=NaHSO4+HBr| ���� ���� | �Ҵ� | ������ | 1��2-�������� | ���� | Ũ���� |
| �ܶ�/g•cm-3 | 0.79 | 1.46 | 2.2 | 0.71 | 1.84 |
| �۵㣨�棩 | -130 | -119 | 9 | -116 | 10 |
| �е㣨�棩 | 78.5 | 38.4 | 132 | 34.6 | 338 |
| ��ˮ�е��ܽ�ȣ�g�� | ���� | 0.914 | 1 | 7.5 | ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
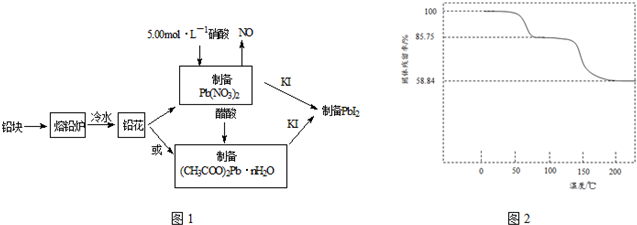
| ʵ������ | ʵ�鷽�� | ʵ������ԭ����� |
| �ٻ�����Ũ�������ƽ���Ӱ�� | ȡPbI2������Һ������һ֧�Թ��У��ٵ��뼸��NaI������Һ | ������Һ��c��I-������ʹQ������PbI2��Ksp |
| ��Ǧ����Ũ�ȼ�С��ƽ���Ӱ�� | ȡPbI2����Һ������һ֧�Թ��У��ټ�������NaCl������Һ | ����ɫ������ʧ ԭ���γ�PbCl42-��������Һ��c��Pb2+����С��ʹQcС��PbI2��Ksp |
| ��Ǧ���Ӻ͵�����Ũ�ȶ���С��ƽ���Ӱ�� | ��PbI2����Һ�е��뼸��FeCl3 ������Һ | ����ɫ������ʧ д����Ӧ�����ӷ���ʽ�� PbI2+2Fe3++4Cl-=PbCl42-+2Fe2++I2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
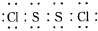 �������ȶ�����ˮ������᪻���Ӧ��һ������Ԫ�ؼ�̬���ߣ�һ���ֽ��ͣ�����Ӧ�漰�ļ������ʵ��۷е������
�������ȶ�����ˮ������᪻���Ӧ��һ������Ԫ�ؼ�̬���ߣ�һ���ֽ��ͣ�����Ӧ�漰�ļ������ʵ��۷е������| ���� | S | CS2 | CCl4 | S2Cl2 |
| �е�/�� | 445 | 47 | 77 | 137 |
| �۵�/�� | 113 | -109 | -23 | -77 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com