

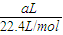 =
=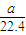 mol���ɷ���ʽAlN+NaOH+H2O=NaAlO2+NH3����֪����Ʒ��AlN�����ʵ���Ϊ=
mol���ɷ���ʽAlN+NaOH+H2O=NaAlO2+NH3����֪����Ʒ��AlN�����ʵ���Ϊ=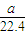 mol������AlN������Ϊ
mol������AlN������Ϊ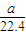 mol×41g/mol=
mol×41g/mol=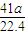 g����Ʒ��AIN����������Ϊ
g����Ʒ��AIN����������Ϊ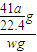 ×100%=
×100%=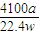 %��
%��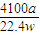 %��
%�� 

 ��ѧ�̸̳����¿α�ϵ�д�
��ѧ�̸̳����¿α�ϵ�д� Сѧ��ʱ��ѵϵ�д�
Сѧ��ʱ��ѵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||

| 41n |
| 17m |
| 41n |
| 17m |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| 4100a |
| 22.4w |
| 4100a |
| 22.4w |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
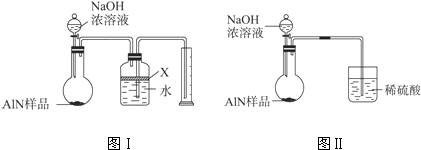
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������AlN����һ���۵�ܸߡ�Ӳ�ȴ����硢������ˮ�������ܼ��ľ��塣�����и������ʼ����ۻ������������˷����Ӽ����������AlN�˷����Ӽ������������ͬ����
A.ˮ�������ʯ
B.ʳ�Σ������
C.�⣬��
D.ʯī����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com