
(16��)�йش����Ĵ��������Դӡ��Ҵ�������ʵ�顱�õ�һЩ��ʶ��ij��ʦ�������ͼװ��(�г�װ��������ʡ��)����ʵ�����Ϊ���Ȱ�ͼ��װ�ã��ȹرջ���a��b��c����ͭ˿���м䲿�ּ���Ƭ�̣�Ȼ�����a��b��c��ͨ�����ƻ���a��b�����н���(��Ъ��)ͨ�����壬������M���۲쵽���Ե�ʵ�������Իش��������⣺
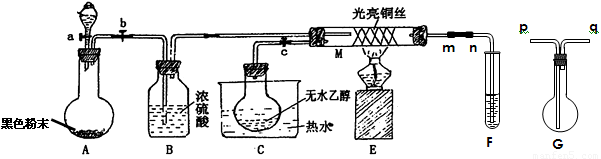
(1)����װ��A�����Եķ����� ��
A�з�����Ӧ�Ļ�ѧ����ʽΪ ��
C����ƿ��������70��80�����ˮ�У�Ŀ���� ��
(2)M���пɹ۲쵽������Ϊ �����п���ʶ����ʵ����̵Ĵ����� ��
(3)���Թ�F����ˮ���ղ����Ҫ�ڵ���m��n֮�����Gװ�ã������ӷ�����(��Gװ���е��ܵĴ���)��m��______��_______��n��Gװ�õ������� �������ﲻ��ˮ���ն���ֱ����ȴ��Ӧ���Թ�F���� �С�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(16��)�йش����Ĵ��������Դӡ��Ҵ�������ʵ�顱�õ�һЩ��ʶ��ij��ʦ�������ͼװ��(�г�װ��������ʡ��)����ʵ�����Ϊ���Ȱ�ͼ��װ�ã��ȹرջ���a��b��c����ͭ˿���м䲿�ּ���Ƭ�̣�Ȼ�����a��b��c��ͨ�����ƻ���a��b�����н���(��Ъ��)ͨ�����壬������M���۲쵽���Ե�ʵ�������Իش��������⣺
(1)����װ��A�����Եķ����� ��
A�з�����Ӧ�Ļ�ѧ����ʽΪ ��
C����ƿ��������70��80�����ˮ�У�Ŀ���� ��
(2)M���пɹ۲쵽������Ϊ �����п���ʶ����ʵ����̵Ĵ����� ��
(3)���Թ�F����ˮ���ղ����Ҫ�ڵ���m��n֮�����Gװ�ã������ӷ�����(��Gװ���е��ܵĴ���)��m��______��_______��n��Gװ�õ������� �������ﲻ��ˮ���ն���ֱ����ȴ��Ӧ���Թ�F���� �С�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��ɽ��ʡ�Ͳ�һ�и�����ѧ����ĩ��⻯ѧ�Ծ� ���ͣ������
(16��)��1����֪:
Fe��s��+1/2O2��g��=FeO��s�� 
2Al��s��+3/2O2��g��= Al2O3��s�� 
Al��FeO�������ȷ�Ӧ���Ȼ�ѧ����ʽ��_____________________________________��
��2����Ӧ�����������Ϊ��̬��ij���淴Ӧ�ڲ�ͬ�����µķ�Ӧ���̷ֱ�ΪA��B������ͼ��ʾ��
�پ�ͼ�жϸ÷�Ӧ��_____(������š�) �ȷ�Ӧ������Ӧ�ﵽƽ��������������䣬�����¶ȣ���Ӧ���ת����__ _ _ (���������С�����䡱)
������B���̱����˷�Ӧ���õ�����Ϊ______ (ѡ�����������ĸ)
A�������¶� B������Ӧ���Ũ��
C�������¶� D��ʹ���˴���
��3��1000��ʱ���������������������з�Ӧ��Na2SO4(s) + 4H2(g)  Na2S(s) + 4H2O(g) ��
Na2S(s) + 4H2O(g) ��
�ٸ÷�Ӧ��ƽ�ⳣ������ʽΪ____________________����֪K1000����K1200������÷�Ӧ��________��Ӧ������ȡ����ȡ�����
�����й����ӷ���ʽ˵��������Ӧ���ù�������ˮ��Һ�������____________
��4�������£����ȡ0.1mol��L��1HA��Һ��0.1mol��L��1NaOH��Һ�������ϣ���Ϻ���Һ����ı仯���Բ��ƣ�����û��Һ��pH��8����ش��������⣺
�ٻ����Һ��ˮ�������c(H��)��0.1mol��L��1NaOH��Һ��ˮ�������c(H��)�Ƚ�
���������������
����֪NH4A��ҺΪ���ԣ���֪��HA��Һ�ӵ�Na2CO3��Һ��������ų������ƶ�(NH4)2CO3��Һ��pH 7�����������������ͬ�¶��£������ʵ���Ũ�ȵ���������Һ��pH�ɴ�С������˳��Ϊ ��������ţ�
a.��NH4HCO3 b��NH4A c��(NH4)2CO3 d��NH4Cl
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�����ʡ����һ�и���������ģ�⿼�ԣ����ۣ���ѧ���� ���ͣ�ʵ����
(16��)�йش����Ĵ��������Դӡ��Ҵ�������ʵ �顱�õ�һЩ��ʶ��ij��ʦ�������ͼװ��(��
�顱�õ�һЩ��ʶ��ij��ʦ�������ͼװ��(�� ��װ��������ʡ��)����ʵ�����Ϊ���Ȱ�ͼ��װ�ã��ȹرջ���a��b��c����ͭ˿���м䲿�ּ���Ƭ�̣�Ȼ�����a
��װ��������ʡ��)����ʵ�����Ϊ���Ȱ�ͼ��װ�ã��ȹرջ���a��b��c����ͭ˿���м䲿�ּ���Ƭ�̣�Ȼ�����a ��b��c��ͨ�����ƻ���a��b�����н���(��Ъ��)ͨ�����壬������M���۲쵽���Ե�ʵ�������Իش��������⣺
��b��c��ͨ�����ƻ���a��b�����н���(��Ъ��)ͨ�����壬������M���۲쵽���Ե�ʵ�������Իش��������⣺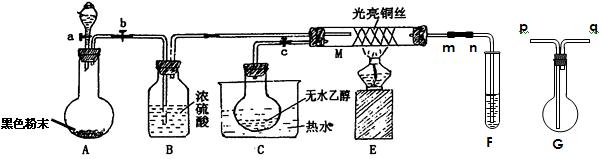
(1)����װ��A�����Եķ����� ��
A�з�����Ӧ�Ļ�ѧ����ʽΪ ��
C����ƿ��������70��80�����ˮ�У�Ŀ���� ��
(2)M���пɹ۲쵽������Ϊ �����п���ʶ����ʵ����̵Ĵ����� ��
(3)���Թ�F����ˮ���ղ�� ��Ҫ�ڵ���m��n֮�����Gװ�ã������ӷ�����(��Gװ���е��ܵĴ���)��m��______��_______��n��Gװ�õ������� �������ﲻ��ˮ���ն���ֱ����ȴ��Ӧ���Թ�F���� �С�
��Ҫ�ڵ���m��n֮�����Gװ�ã������ӷ�����(��Gװ���е��ܵĴ���)��m��______��_______��n��Gװ�õ������� �������ﲻ��ˮ���ն���ֱ����ȴ��Ӧ���Թ�F���� �С�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ɽ��ʡ������ѧ����ĩ��⻯ѧ�Ծ� ���ͣ������
(16��)��1����֪:
Fe��s��+1/2O2��g��=FeO��s�� 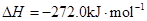
2Al��s��+3/2O2��g��= Al2O3��s�� 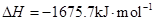
Al��FeO�������ȷ�Ӧ���Ȼ�ѧ����ʽ��_____________________________________��
��2����Ӧ�����������Ϊ��̬��ij���淴Ӧ�ڲ�ͬ�����µķ�Ӧ���̷ֱ�ΪA��B������ͼ��ʾ��

�پ�ͼ�жϸ÷�Ӧ��_____(������š�) �ȷ�Ӧ������Ӧ�ﵽƽ��������������䣬�����¶ȣ���Ӧ���ת����__ _ _ (���������С�����䡱)
������B���̱����˷�Ӧ���õ�����Ϊ______ (ѡ�����������ĸ)
A�������¶� B������Ӧ���Ũ��
C�������¶� D��ʹ���˴���
��3��1000��ʱ���������������������з�Ӧ��Na2SO4(s)
+ 4H2(g)  Na2S(s)
+ 4H2O(g) ��
Na2S(s)
+ 4H2O(g) ��
�ٸ÷�Ӧ��ƽ�ⳣ������ʽΪ____________________����֪K1000����K1200������÷�Ӧ��________��Ӧ������ȡ����ȡ�����
�����й����ӷ���ʽ˵��������Ӧ���ù�������ˮ��Һ�������____________
��4�������£����ȡ0.1mol��L��1HA��Һ��0.1mol��L��1NaOH��Һ�������ϣ���Ϻ���Һ����ı仯���Բ��ƣ�����û��Һ��pH��8����ش��������⣺
�ٻ����Һ��ˮ�������c(H��)��0.1mol��L��1NaOH��Һ��ˮ�������c(H��)�Ƚ�
���������������
����֪NH4A��ҺΪ���ԣ���֪��HA��Һ�ӵ�Na2CO3��Һ��������ų������ƶ�(NH4)2CO3��Һ��pH 7�����������������ͬ�¶��£������ʵ���Ũ�ȵ���������Һ��pH�ɴ�С������˳��Ϊ ��������ţ�
a.��NH4HCO3 b��NH4A c��(NH4)2CO3 d��NH4Cl
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com