
![]() ��ȡ�����У��¶���Ѹ������
��ȡ�����У��¶���Ѹ������
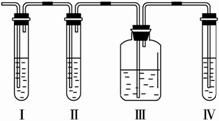
��1����װ��ʢ�ŵ��Լ��ǣ���.__________����.__________����.__________����.__________�������й��Լ���������ں����ϣ�
A.Ʒ�� B. NaOH C.ŨH2SO4 D.KMnO4������Һ
��2����˵��SO2������ڵ�������__________��
��3��ʹ��װ�â��Ŀ����__________��ʹ��װ�â��Ŀ����__________��
��4��ȷ�Ϻ���ϩ��������__________��
˼·�������¶ȹ��ߣ���ʹ�ƾ�̼����Ȼ������ӦC+2H2SO4��Ũ��![]() CO2��+2SO2��+2H2O��ʹ��ϩ�л���SO2��CO2���壬��Ӱ����ϩ�ļ��顣����Ӧ������Ʒ������SO2����ͨ����Һ��ȥSO2��������֤��ϩ�Ĵ��ڡ�
CO2��+2SO2��+2H2O��ʹ��ϩ�л���SO2��CO2���壬��Ӱ����ϩ�ļ��顣����Ӧ������Ʒ������SO2����ͨ����Һ��ȥSO2��������֤��ϩ�Ĵ��ڡ�
�𰸣���ֹ����Ӧ���� C2H5OH+C2H5OH![]() C2H5��O��C2H5+H2O
C2H5��O��C2H5+H2O
��1��A B A D
��2������Ʒ����Һ��ɫ
��3���Ա�֤��ȥSO2�� CO2 ����SO2�Ƿ������
��4������KMnO4������Һ��ɫ


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
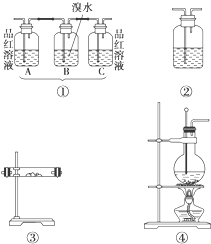 ʵ��������ϩ�ķ�Ӧԭ��ΪCH3CH2OH
ʵ��������ϩ�ķ�Ӧԭ��ΪCH3CH2OH| Ũ���� | 170�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ������2012��2013ѧ���һ��ѧ����ĩ����ģ������(2)����ѧ ���ͣ�058
ij��ѧ����С��������Ϸ��֣�ʵ��������ϩ�ķ�Ӧԭ��ΪCH3CH2OH![]() CH2
CH2![]() CH2����H2O���Ƶõ���ϩ����������CO2��SO2��ˮ����������������װ�����һ��ʵ�飬����֤��ϩ��������������ijɷ֣�
CH2����H2O���Ƶõ���ϩ����������CO2��SO2��ˮ����������������װ�����һ��ʵ�飬����֤��ϩ��������������ijɷ֣�
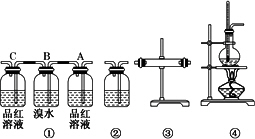
��ش��������⣺
(1)װ�õ�����˳��(���������������ҵ�����)Ϊ________��
(2)װ�â��У�Aƿ�е�������________������Ϊ________��Bƿ�е�������________��Bƿ�е�����Ϊ________����Cƿ��Ʒ����Һ����ɫ���ɵõ��Ľ�����________��
(3)װ�â��м���Ĺ���ҩƷ��________��Ŀ������֤��������к���________��װ�â���ʢװ����Һ��________��Ŀ������֤������к���________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ͬ���� ���ͣ�ʵ����
 CH2=CH2��+H2O����ȡʱ�������¶ȹ��߶���������Ӧ��ʹ�����Ҵ���Ũ���ᷴӦ���ɶ�����������̼��ˮ������̿�ڡ�
CH2=CH2��+H2O����ȡʱ�������¶ȹ��߶���������Ӧ��ʹ�����Ҵ���Ũ���ᷴӦ���ɶ�����������̼��ˮ������̿�ڡ� 
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com