
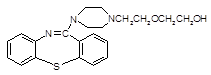 )2��
)2��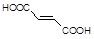 �����ĺϳ�·�����£�
�����ĺϳ�·�����£�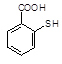
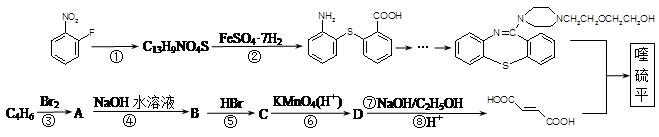
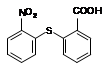 �г����Ѽ�����S�����⣬�����еĹ���������Ϊ �� ��
�г����Ѽ�����S�����⣬�����еĹ���������Ϊ �� ��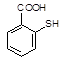 һ���������γɾۺ���Ľṹ��ʽΪ ��
һ���������γɾۺ���Ľṹ��ʽΪ �� HOCH2CH��CHCH2OH��2NaBr
HOCH2CH��CHCH2OH��2NaBr 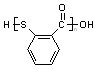
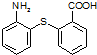 ��֪C13H9NO4S�����ʵĽṹ��ʽΪ
��֪C13H9NO4S�����ʵĽṹ��ʽΪ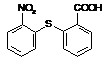 ��
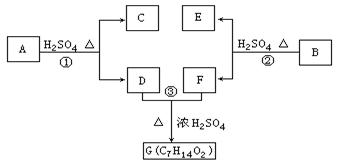
�� �л�����-NO2��-COOH�����ţ��ֱ�Ϊ�������Ȼ�����2��C4H6����ӦΪCH2=CH-CH=CH2�����巢��1��4�ӳɣ�����CH2Br-CH=CH-CH2Br����Ӧ�ݵ�Ŀ���Ƿ�ֹC=C�����Ը���������������������ŵ����ã��ʴ�Ϊ���ӳɷ�Ӧ������̼̼˫������ֹ������KMnO4��������3��AΪCH2Br-CH=CH-CH2Br���ڼ��������·���ȡ����Ӧ����HOCH2CH=CHCH2OH����Ӧ�Ļ�ѧ����ʽΪBrCH2CH��CHCH2Br��2NaOH
�л�����-NO2��-COOH�����ţ��ֱ�Ϊ�������Ȼ�����2��C4H6����ӦΪCH2=CH-CH=CH2�����巢��1��4�ӳɣ�����CH2Br-CH=CH-CH2Br����Ӧ�ݵ�Ŀ���Ƿ�ֹC=C�����Ը���������������������ŵ����ã��ʴ�Ϊ���ӳɷ�Ӧ������̼̼˫������ֹ������KMnO4��������3��AΪCH2Br-CH=CH-CH2Br���ڼ��������·���ȡ����Ӧ����HOCH2CH=CHCH2OH����Ӧ�Ļ�ѧ����ʽΪBrCH2CH��CHCH2Br��2NaOH HOCH2CH��CHCH2OH��2NaBr;��4��BΪOH-CH2-CH=CH-CH2-OH���Ⱥ����ǻ����ֺ���ȩ����ͬ���칹���У�CH2��OH��CH2CH2CHO��CH3CH��OH��CH2CHO��CH3CH2CH��OH��CHO��HOCH2CH��CH3��CHO��CH2C��OH����CH3��CHO�����Թ���5�֣���5������
HOCH2CH��CHCH2OH��2NaBr;��4��BΪOH-CH2-CH=CH-CH2-OH���Ⱥ����ǻ����ֺ���ȩ����ͬ���칹���У�CH2��OH��CH2CH2CHO��CH3CH��OH��CH2CHO��CH3CH2CH��OH��CHO��HOCH2CH��CH3��CHO��CH2C��OH����CH3��CHO�����Թ���5�֣���5������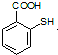 ��һ�������·������۷�Ӧ��������������Ľṹ��ʽΪ��
��һ�������·������۷�Ӧ��������������Ľṹ��ʽΪ��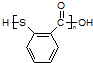 ��
��

 �����������Ů��ͯ������ϵ�д�
�����������Ů��ͯ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
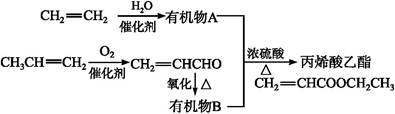
 CH2�Ƶ��л���A�Ļ�ѧ����ʽΪ������������������,��Ӧ����������������
CH2�Ƶ��л���A�Ļ�ѧ����ʽΪ������������������,��Ӧ����������������  CH2)�к��еĹ�������������(������)��
CH2)�к��еĹ�������������(������)�� �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ƶ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
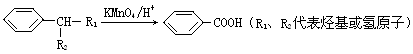
 RCH
RCH C��COOH��2+H2O��RCH
C��COOH��2+H2O��RCH C��COOH��2
C��COOH��2 RCH
RCH CHCOOH+CO2����
CHCOOH+CO2����| A��������Ӧ | B��ˮ�ⷴӦ |
| C����ȥ��Ӧ | D��������Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ƶ���
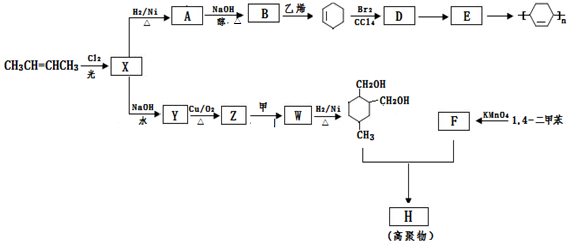

 ��Z��W ��
��Z��W ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
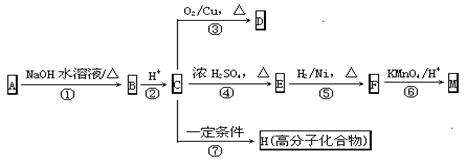
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
|
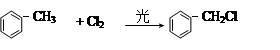 �� R��CH2��CH = CH2 + Cl2
�� R��CH2��CH = CH2 + Cl2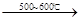 R��CHCl��CH = CH2 + HCl
R��CHCl��CH = CH2 + HCl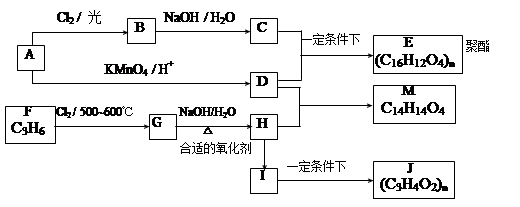
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
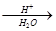 R-COOH��E������������ˮ���IJ�����һ�������¿�����F��C11H10O3����д��F�Ľṹ��ʽ�� ��
R-COOH��E������������ˮ���IJ�����һ�������¿�����F��C11H10O3����д��F�Ľṹ��ʽ�� ��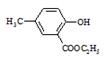 �ĺϳ�·������ͼ��
�ĺϳ�·������ͼ�� CH3CH2OH
CH3CH2OH  H2C��CH2 BrH2C��CH2Br
H2C��CH2 BrH2C��CH2Br �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ƶ���
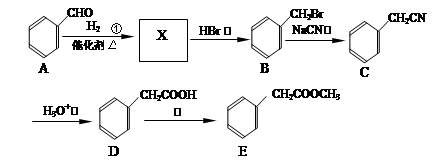
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com