



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ȼ�ŵľƾ��Ʋ�������ʧ��Ӧ��Ѹ����ʪĨ���˸� |
| B���õ�����ƽ������ѧҩƷʱ�������ȳ�С�ձ����������ٳ��������Լ����������������֮�ΪҩƷ�������� |
| C���Ʊ�Ħ���εĹ����У�ϴ����������茶���ʱ��Ӧ�������ƾ�ϴȥ������渽�ŵ�ˮ�� |
| D���������Ȼ��ܵ��Ҵ���Һ�У���μ���ˮ����Һǡ�óʷۺ�ɫ�����ȸ���Һ��������γ�����ɫ������ɫ����ɫ�ı仯 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����������Ũ���������¼��ȿ��Ƶ���ϩ. |
| B�����Ʊ���������ʵ���У������������������͵��������ã�����̼������ҺҺ��������״������ζҺ������ |
| C��ʵ���ҿ�����ˮ�Ҵ�������3mol/L����Ļ��Һ����ϩ |
| D���õ�ʯ��ˮ���Ƶô�������Ȳ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ⶨ��ҺpH�IJ�������pH��ֽ���ڱ������ϣ�������ˮ��ʪ�����ýྻ������պȡ��Һ������pH��ֽ���в������Ӧ�ı���ɫ���Ƚ� |
| B����ȡ����ʱ��Ӧѡ���л���ȡ��������ȡ�����ܶȱ����ˮ�� |
| C���ⶨ�����м�ȩ������ʹ50mLע��������������ͨ�뵽2mL2��10-4mol/L�������ữ�ĸ��������Һ�У��ظ�ǰ��IJ���ֱ���Ϻ�ɫ��ȥ����¼���Ŀ�����������ɴ��Բⶨ�����м�ȩ������ |
| D����Al��CuSO4��Һ���û���Ӧʵ��ʱ���ɽ�����ֱ�Ӳ���CuSO4��Һ�У��۲���Ƭ�����Ƿ���ͭ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| ���� | Al | Al2O3 | Fe | Fe2O3 |
| �۵�/�� | 660 | 2054 | 1535 | 1462 |
| �е�/�� | 2467 | 2980 | 2750 | ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
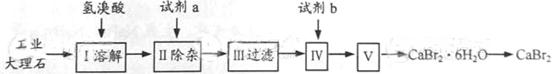
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ۢܢ� | B���ڢۢ� | C���٢ڢ� | D���ܢݢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ij��Һ�м���AgNO3��Һ�а�ɫ�����������ټ����ᣬ��������ʧ�������Һ��һ����Cl�� |
| B������ϡ���������ɫ���壬������ͨ�����ʯ��ˮ�У���Һ����ǣ�һ����CO32�� |
| C������ɫ��ӦǰӦ�Ƚ���˿��ϡ������ϴ�ɾ���պȡ����Һ����ʵ�� |
| D����������������Һ�����ȣ�������������ʹʪ���ɫʯ����ֽ������һ����NH4�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com