
(6��)��1���л���ѧ�еķ�Ӧ���ͽ϶࣬�����з�Ӧ����(�����)��
��������ϩ��PVC ������������������Ҵ���Һ���� ����ϩ������ط�Ӧ ���ɸ������������� ��������Ӧ ���ɱ�������TNT �߱��Ӻ�Ũ��ˮ��Ӧ ��ʵ��������ϩ ����֬Ӳ��
��������ȡ����Ӧ���� ������������Ӧ���� ��
��2�� 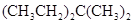 ��ϵͳ����Ϊ ��
��ϵͳ����Ϊ ��
��3��̼̼˫�������Ը�����������£��������±仯��
�� �����������·�����Ӧ���õ������ղ���ṹʽ�ֱ��ǣ�
�����������·�����Ӧ���õ������ղ���ṹʽ�ֱ��ǣ�
��1��
��4��ij�л���ṹ��ʽ��ͼ����Na��NaOH��NaHCO3������ʵ����ĸ��л�����ȫ��Ӧʱ������Na��NaOH��NaHCO3�����ʵ���֮��Ϊ 
����6�֣���1���ܢޢ� �ۢ� ����1�֣�
��2��3,3-�������飨2�֣�
��3��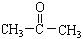
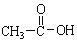 ��4��3:2:1
��4��3:2:1
���������������1�����Ǽӳɷ�Ӧ��������ȥ��Ӧ������������Ӧ������ȡ����Ӧ������������Ӧ������ȡ����Ӧ������ȡ����Ӧ�������Ҵ���ȡ��ϩ�ķ�Ӧ��������ȥ��Ӧ������֬��Ӳ���Ǽӳɷ�Ӧ����������ȡ����Ӧ���Тܢޢߣ�����������Ӧ���Тۢݣ�
��2���������������������л�����̼����5��Cԭ�ӣ�����3��Cԭ������2��ȡ�����Ǽ������Ը��л����������3,3-�������飻
��3������������Ϣ����֪̼̼˫�������Ը������������̼̼˫�����ѣ�Ȼ������-C=O���ɣ�����2-��-2��ϩ��������IJ����DZ�ͪ����ȩ����ȩ���������������ᣬ�ṹ��ʽΪ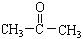 ��
��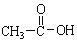 ��
��
��4���ǻ����Ʒ�Ӧ�����ǻ����Ȼ���NaOH��Ӧ��ֻ���Ȼ���NaHCO3��Ӧ�����Ե����ʵ����ĸ��л�����Na��NaOH��NaHCO3��ȫ��Ӧʱ������Na��NaOH��NaHCO3�����ʵ���֮����3:2:1.
���㣺���鷴Ӧ���͵��жϣ��л����������������Ϣ���������ǻ��Ļ��Ե�Ӧ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����������ȷ���� �� ��
| A����ϩ��������������е�����ԭ�Ӷ���ͬһƽ���� |
| B�������ʡ����ۡ���ά�غ���֬�����ڸ߷��ӻ����һ�������¶���ˮ�� |
| C�������������Ӧ����һ�ȼ��飬�뱽�����ᷴӦ�����������ķ�Ӧ������ͬ |
| D���ױ��ȿ�ʹ������Ȼ�̼��Һ��ɫ��Ҳ��ʹ���Ը��������Һ��ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
Ϊ�ⶨij�л�������A�Ľṹ����������ʵ�飺
(һ)����ʽ��ȷ����
��1�����л���A�����������г��ȼ�գ�ʵ���ã�����5.4 g H2O��8.8 g CO2����������6.72 L(��״����)����������и�Ԫ�ص�ԭ�Ӹ�������________��
��2���������Dzⶨ���л����������Է����������õ���ͼ1��ʾ����ͼ��������Է�������Ϊ________�������ʵķ���ʽ��________��
��3�����ݼۼ����ۣ�Ԥ��A�Ŀ��ܽṹ��д���ṹ��ʽ______________________��
(��)�ṹʽ��ȷ����
��4���˴Ź��������ܶ��л�������в�ͬλ�õ���ԭ�Ӹ�����ͬ�ķ�ֵ(�ź�)�����ݷ�ֵ(�ź�)����ȷ����������ԭ�ӵ��������Ŀ�����磺���ȼ���(Cl��CH2��O��CH3)��������ԭ����ͼ2�����ⶨ���л���A�ĺ˴Ź�������ʾ��ͼ��ͼ3����A�Ľṹ��ʽΪ________��
ͼ1 ͼ2 ͼ3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��13�֣��л��ϳ�������̼����һ����Ҫ���ڡ������з�Ӧ��
��  ͨ������·�߿ɺϳɣ���
ͨ������·�߿ɺϳɣ���
��1�����ķ���ʽΪ �����Ľṹ��ʽΪ ��
��2����������������NaOH��Һ��Ӧ�Ļ�ѧ����ʽΪ ��
��3�� �����ɣ���ʱ�����ܵõ���һ�ָ�����C6H8O4���÷�Ӧ�Ļ�ѧ����ʽΪ ����Ӧ������ ��
�����ɣ���ʱ�����ܵõ���һ�ָ�����C6H8O4���÷�Ӧ�Ļ�ѧ����ʽΪ ����Ӧ������ ��
��4������һ��ͬ���칹�壨�����ܷ���������Ӧ������ˮ�����ɲ������ķ��㻯����������Ľṹ��ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��8�֣�����ݹ����ŵIJ�ͬ�������л�����з��ࣺ
��1�����ڷ������� ����2�����ڴ������ ��
��3������ȩ������ ����4������������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(8��)�л���A��B��C��D���������ʡ�
��1������֧���Ļ�����A�ķ���ʽΪC4H6O2��A����ʹBr2�����Ȼ�̼��Һ��ɫ��1molA
��1mol NaHCO3����ȫ��Ӧ����A�Ľṹ��ʽ�� ��
д����A������ͬ�����ŵ�A������ͬ���칹��Ľṹ��ʽ
��
��2��������B����C��H��O����Ԫ�أ�������Ϊ60������̼����������Ϊ60%�������������Ϊ13.33%��B�ڴ���Cu�������±�������C��C�ܷ���������Ӧ����B�Ľṹ��ʽ�� ��
��3��D��NaOHˮ��Һ�м��ȷ�Ӧ��������A�����κ�B����Ӧ��Ӧ�Ļ�ѧ����ʽ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������ABS��֬���ṹ��ʽ���£��ϳ�ʱ�������ֵ��塣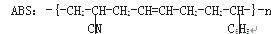
ʽ��- C6H5�DZ����������ֵ���Ľṹ��ʽ�ֱ��ǣ�
�� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com