
 ��Ӣ���㿨ϵ�д�
��Ӣ���㿨ϵ�д� Ӧ����㲦ϵ�д�
Ӧ����㲦ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

 CO2+H2
CO2+H2 2NH3(g)����H<0���±�Ϊ��ͬ�¶��¸÷�Ӧ��ƽ�ⳣ�����ɴ˿���֪������
2NH3(g)����H<0���±�Ϊ��ͬ�¶��¸÷�Ӧ��ƽ�ⳣ�����ɴ˿���֪������| T/K | T1 | 573 | T2 |
| K | 1.00��107 | 2.45��105 | 1.88��103 |
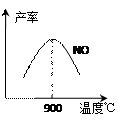 ��3��NH3��O2�ڲ�ϵ���������´�145��Ϳ�ʼ��Ӧ��
��3��NH3��O2�ڲ�ϵ���������´�145��Ϳ�ʼ��Ӧ�� 4NO(g)+6H2O(g)����H=��905kJ��mol��1
4NO(g)+6H2O(g)����H=��905kJ��mol��1�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��1���ʰ�������д���9�Թ��õ��� |
| B��PCl3��BCl3����������ԭ�ӵ�����㶼�ﵽ8�����ȶ��ṹ |
| C��H2S��CS2���Ӷ��Ǻ����Լ��ļ��Է��� |
| D���۵��ɸߵ��͵�˳���ǣ����ʯ��̼���裾����� |
�鿴�𰸺ͽ���>>
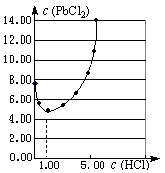
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ���� ����ʽ��HCl ��Է���������36.5 �ܶȣ�1.19g/m3 ����������36.5% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ڢݢޡ��� | B���ڢܢޡ� | C���٢ۢݡ��� | D���٢ۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
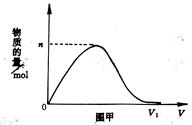
| A��4��3 | B��7��3 |
| C��3��5 | D��3��7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
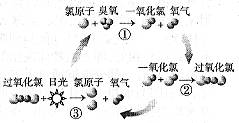
| A���չ��ڷ�Ӧ�������� |
| B������������һ�ֺ��ȶ������� |
| C���������ȵĽṹʽΪO��Cl��Cl��O |
| D��������������ת����������� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com