
| 100mL���� | 100mL���� | 100mL���� | |
| m������ | 9.2g | 15.7g | 27.6g |
| V��CO2������״���� | 2.24L | 3.36L | 3.36L |
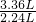 ��9.2g=13.8g�����Եڶ���ʵ�����β��㣬�������ʣ�࣮
��9.2g=13.8g�����Եڶ���ʵ�����β��㣬�������ʣ�࣮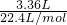 =0.15mol���ɷ���ʽ��֪n��HCl��=n��CO2�����ݴ˼��㣻
=0.15mol���ɷ���ʽ��֪n��HCl��=n��CO2�����ݴ˼��㣻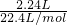 =0.1mol����9.2 g�������NaHCO3��KHCO3�����ʵ����ֱ�Ϊxmol��ymol������̼Ԫ���غ��֪n��CO2��=n��NaHCO3��+n��KHCO3���������û���������з��̼���x��y��ֵ����������������NaHCO3��KHCO3�������ȣ�
=0.1mol����9.2 g�������NaHCO3��KHCO3�����ʵ����ֱ�Ϊxmol��ymol������̼Ԫ���غ��֪n��CO2��=n��NaHCO3��+n��KHCO3���������û���������з��̼���x��y��ֵ����������������NaHCO3��KHCO3�������ȣ�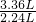 ��9.2g=13.8g�����Եڶ���ʵ�����β��㣬�������ʣ�࣮
��9.2g=13.8g�����Եڶ���ʵ�����β��㣬�������ʣ�࣮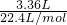 =0.15mol���ɷ���ʽ��֪n��HCl��=n��CO2��=0.15mol����Ԫ�ص����ʵ���Ũ��Ϊ
=0.15mol���ɷ���ʽ��֪n��HCl��=n��CO2��=0.15mol����Ԫ�ص����ʵ���Ũ��Ϊ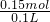 =1.5mol/L����A����
=1.5mol/L����A����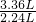 ��9.2g=13.8g��15.7�ˣ����Ի�������������㣬��B��ȷ��
��9.2g=13.8g��15.7�ˣ����Ի�������������㣬��B��ȷ��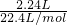 =0.1mol��������ӦH++HCO3-=CO2��+H2O���ɷ���ʽ��֪�����ĵ��������ʵ���Ϊ0.1mol����C��ȷ��
=0.1mol��������ӦH++HCO3-=CO2��+H2O���ɷ���ʽ��֪�����ĵ��������ʵ���Ϊ0.1mol����C��ȷ��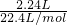 =0.1mol����9.2 g�������NaHCO3��KHCO3�����ʵ����ֱ�Ϊxmol��ymol������̼Ԫ���غ��֪��x+y=0.1mol�����������Ϊ9.2g������84x+100y=9.2g���������̽��x=0.05 mol��y=0.05 mol���ʻ������NaHCO3��KHCO3��������Ϊ84��100=21��25����D����
=0.1mol����9.2 g�������NaHCO3��KHCO3�����ʵ����ֱ�Ϊxmol��ymol������̼Ԫ���غ��֪��x+y=0.1mol�����������Ϊ9.2g������84x+100y=9.2g���������̽��x=0.05 mol��y=0.05 mol���ʻ������NaHCO3��KHCO3��������Ϊ84��100=21��25����D����


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 CO2��SO2��NOx����Ҫ�ķǽ�������������ǵ����滷��ϢϢ��أ�
CO2��SO2��NOx����Ҫ�ķǽ�������������ǵ����滷��ϢϢ��أ�| C(HCO3-)C(OH-) |
| C(CO32-) |
| C(HCO3-)C(OH-) |
| C(CO32-) |
| 5 |
| 144 |
| 5 |
| 144 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 CO2��SO2��NOx����Ҫ�ķǽ�������������ǵ����滷��ϢϢ��أ�
CO2��SO2��NOx����Ҫ�ķǽ�������������ǵ����滷��ϢϢ��أ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
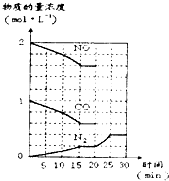
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��ɽ��ʡ�Ͳ�һ�и߿�ģ�⻯ѧ�Ծ���4�·ݣ��������棩 ���ͣ������

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com