
����ͼװ�ý���ʵ�飬��֪C1��C2Ϊʯī����
�ش���������:
��1���ж�װ�õ����ƣ�A��Ϊ�ߣߣߣߣߣߣߣ�B��Ϊ�ߣߣߣߣߣߣߣߣ�
��2��п��Ϊ�ߣߣߣߣ����缫��ӦʽΪ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�
ͭ��Ϊ�ߣߣߣߣ����缫��ӦʽΪ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�
ʯī��C1Ϊ�ߣߣ����缫��ӦʽΪ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�
ʯī��C1����������ʵ������Ϊ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��3����C1������224mL���壨��״���£�ʱ��п�������ߣߣߣߣ�����ӡ��������䡱���١����ˣߣߣߣߣ�g��CuSO4��Һ�������ߣߣߣߣ�����ӡ��������䡱���١����ߣߣߣߣ�g��
 ���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ij����С��Լ�����������µ�ʵ�飬����֤�京��ȩ�����������仯ѧ���ʣ���������������Ӧ��
ij����С��Լ�����������µ�ʵ�飬����֤�京��ȩ�����������仯ѧ���ʣ���������������Ӧ�� ��CH3CH2-18OH����������Ӧ�Ļ�ѧ����ʽ
��CH3CH2-18OH����������Ӧ�Ļ�ѧ����ʽ| ŨH2SO4 |
| ���� |
| ŨH2SO4 |
| ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| �� |
| ||
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�������ʡ�������е�����ѧ������ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
��14�֣�ijУ��ѧ�о���ѧϰС���������ʵ�鷽�����ⶨ�����Ѿõ�С�մ���Ʒ�д��������������
��1������һ����ȡһ����������Ʒ�����������м��������غ���ȴ������ʣ��������������㡣ʵ���м��������ص�Ŀ���� ��
��2��������������ͼװ�ý���ʵ�顣���ش��������⣺
��ʵ��ǰӦ��________________����Һ©����Ӧ��װ___________������ᡱ��ϡ�����Ρ�����Dװ�õ�������_________________________________________��
��ʵ���г�������Ʒ�����⣬�����________װ�ã�����ĸ��ʾ��ǰ�������ı仯��
�۸��ݴ�ʵ��õ������ݣ��ⶨ����нϴ�����Ϊʵ��װ�û�����һ������ȱ�ݣ���ȱ���� ��
��3������������ȡһ������Ʒ������С�ձ��У�������ˮ�ܽ⣬��С�ձ��м��������Ȼ�����Һ������ϴ�ӣ���������������������������㣺
�ٹ��˲����У������ձ���©����õ��IJ���������______________________��
���������жϳ����Ƿ���ȫ�ķ�����_______________________________________
���������Լ���Ϊ������������֪�Ƶ���Ʒ9.5g������ij�������Ϊ19.7g������Ʒ��̼���Ƶ���������Ϊ_________________������һλС������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���ս̰���л�ѧѡ��5 1.2��ѧ�������о��л�����ϰ���������棩 ���ͣ�ʵ����
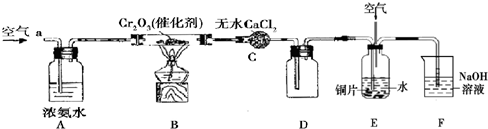
ʵ������ȼ�շ��ⶨij�ְ�����(CxHyOz?Np)�ķ�����ɣ�ȡmg���ְ�������ڴ����г��ȼ�ա�����CO2��H2O��N2���ְ���ͼװ�ý���ʵ�飬������������⣺

(1)ʵ�鿪ʼʱ������Ҫͨ��һ��ʱ�����������������__________________________��
(2)����װ������Ҫ���ȵ�������________(����ĸ)������ʱӦ�ȵ�ȼ______���ľƾ��ơ�
(3)Aװ���з�����Ӧ�Ļ�ѧ����ʽΪ____________________________________��
(4)װ��D��������_______________________________��
(5)��ȡN2�����ʱ��Ӧע���__________________����___________________________��
(6)ʵ���в��N2�����ΪVmL(������ɱ�״��)��Ϊȷ���˰�����ķ���ʽ������Ҫ���й�������__________��
A������CO2���������
B������ˮ������
C��ͨ��O2�����
D�����������Է�������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com