��1913�깤ҵ�ϳɰ�Ͷ���������ϳɰ���ҵ���Ϸ�չ�����ֿ��Խ�һ���Ʊ����ᣬ�ڹ�ҵ�Ͽɽ���������������ش��������⣺
��1����֪��N
2��g��+O
2��g��=2NO��g����H=+180.5kJ/molN
2��g��+3H
2��g��?2NH
3��g����H=-92.4kJ/mol2H
2��g��+O
2��g��=2H
2O��g����H=-483.6kJ/mol
д������������������һ�����������ˮ�������Ȼ�ѧ����ʽ��
4NH3��g��+5O2��g���T4NO��g��+6H2O��g����H=-905.0kJ/mol
4NH3��g��+5O2��g���T4NO��g��+6H2O��g����H=-905.0kJ/mol
��
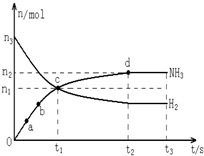
��2��ij�о�С����673K��30MPa�����£������ΪVL���ܱ������н��з�Ӧ��
N
2��g��+3H
2��g��?2NH
3��g������n��H
2����n��NH
3����ʱ��仯�Ĺ�ϵ��ͼ��ʾ��
����������ȷ����
AC
AC
��������ĸ��
A����a������Ӧ���ʱȵ�b�Ĵ�
B����c����Ӧ�ﵽ��ѧƽ��״̬
C����t
3ʱ��673K����773K����n��H
2��������
D��t
2��t
3ʱ�̣�n��N
2�������
��3����һ���¶Ⱥʹ����£���6.4mol H
2��2.4molN
2�����һ���ݻ�Ϊ4L���ܱ������з�����Ӧ����3minĩʱ��Ӧǡ�ô�ƽ�⣬��ʱ������1.6mol NH
3�����㣺��д��������̣���3min����H
2��ʾ�Ļ�ѧ��Ӧ���ʣ��ڸ������µ�ƽ�ⳣ����

 ��1913�깤ҵ�ϳɰ�Ͷ���������ϳɰ���ҵ���Ϸ�չ�����ֿ��Խ�һ���Ʊ����ᣬ�ڹ�ҵ�Ͽɽ���������������ش��������⣺
��1913�깤ҵ�ϳɰ�Ͷ���������ϳɰ���ҵ���Ϸ�չ�����ֿ��Խ�һ���Ʊ����ᣬ�ڹ�ҵ�Ͽɽ���������������ش��������⣺ 2NH3��g��
2NH3��g��


