
��һ��(4��)�����е����������ʣ�д����ȥ��Щ���ʵ��Լ���
��1��MgO (Al2O3) ��2��Cl2(HCl)
��3��FeCl3(FeCl2) ��4��NaHCO3��Һ(Na2CO3)
������(6��)��ˮ�к��д������Ȼ�þ���Ӻ�ˮ����ȡþ��������������ͼ��ʾ��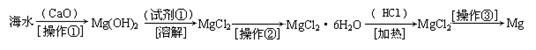
�ش��������⣺
д���ں�ˮ�м�������������������þ�Ļ�ѧ����ʽ ��
��������Ҫ��ָ ���Լ��ٿ�ѡ�� ��
��������ָ �������������տɵý���þ��
��������8�֣�ʵ��������480ml 0��1mol��L-1��Na2CO3��Һ���ش��������⣺
��1��Ӧ��������ƽ��ȡʮˮ̼���ƾ��� g��
��2����ͼ��ʾ������������Һ�϶�����Ҫ���� (�����)����ʵ�����貣������E���Ϊ mL��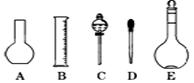
��3������ƿ�ϱ��У����¶ȡ���Ũ�ȡ�����������ѹǿ���ݿ̶��ߡ�����ʽ���ʽ�������е� ���������ַ��ţ�
��4�������������Ҫ�����ǣ�a����ƿ��b�ձ���c��ͷ�ιܡ�d������ƽ�������ڲ���������ʹ�õ�ǰ��˳���� ������д��ĸ��ÿ������ֻ��ѡ��һ�Σ�
��5���������ǻ�ѧʵ���г��õ�һ�ֲ������ߣ�����������Һ�Ĺ����в����������� ����;������д���֣�
��6����ʵ��ʱ���������������ʹ��Һ��Ũ��ƫ�͵��� ��
| A������ǰû�н�����ƿ�е�ˮ������ |
| B��̼����ʧȥ�˲��ֽᾧˮ�� |
| C��̼���ƾ��岻�������л����Ȼ��ƣ� |
| D������̼���ƾ���ʱ�����������⣻ |
��һ����4�֣���1�� NaOH��Һ��2������NaCl��Һ��3��Cl2��4��CO2��ÿ��1�֣�
��������6�֣�CaO+H2O+MgCl2=Mg(OH)2+CaCl2��2�֣����ˣ�1�֣� HCl��1�֣�
����Ũ������ȴ�ᾧ�����ˣ�2�֣�
(��)��8�֣���1��14��3����2��AC 500 ��3���٢ۢ�
��4��dbac ��5��2 (6)CE ���ڣ�6����2�֣�����ÿ��1�֣���8�֣�
���������������һ����1������Al2O3Ϊ�������������NaOH��Һ�ɳ�ȥMgO�е�Al2O3���ʡ�
��2��Cl2�ڱ���NaCl��Һ�е��ܽ�Ⱥ�С�������ñ���NaCl��Һ�ɳ�ȥCl2�е�HCl��
��3��Cl2�ɰ�FeCl2����ΪFeCl3������ͨ��Cl2�ɳ�ȥFeCl2��
��4��CO2��H2O��Na2CO3��Ӧ����NaHCO3������CO2�ɳ�ȥNaHCO3�е�Na2CO3���ʡ�
����������CaO��H2O��Ӧ����Ca(OH)2��CaO+H2O=Ca(OH)2��Ȼ��Ca(OH)2��MgCl2��Ӧ����Mg(OH)2��Ca(OH)2+MgCl2=Mg(OH)2+CaCl2���ӺͿɵ��ܷ���ʽ��CaO+H2O+MgCl2=Mg(OH)2+CaCl2�������ٰ�Mg(OH)2�������������Ϊ���ˣ��Լ�����Mg(OH)2��Ӧ����MgCl2��ΪHCl���������Ǵ�MgCl2��Һ�еõ�MgCl2��6H2O��Ϊ����Ũ������ȴ�ᾧ�����ˡ�
��������1������480ml��Ҫѡ��500ml����ƿ��m(Na2CO3��10H2O)=0��5L��0��1mol/L��286g/mol=14��3g��
��2������һ�����ʵ���Ũ�ȵ���Һ���ò�����ƿ����Һ©������������ƿ�Ĺ��ʵ����������ƿΪ500ml��
��3������ƿ�ϱ����¶ȡ������Ϳ̶��ߣ��ʴ�Ϊ�٢ۢݡ�
��4��������������ƽ�������ʣ�Ȼ������ʷ����ձ��У���ˮ�ܽ⣬����Һע������ƿ������ý�ͷ�ιܶ��ݣ������ڲ���������ʹ�õ�ǰ��˳����dbac��
��5���ܽ�����ʱ�ò��������裬ת����Һʱ�ò��������������Բ�����������2����;��
��6��A�� ����ǰû�н�����ƿ�е�ˮ��������Ũ����Ӱ�죻B��̼����ʧȥ�˲��ֽᾧˮ��Na2CO3���Ũ��ƫ�ߣ�C��̼���ƾ��岻�������л����Ȼ��ƣ�������Na2CO3���٣�Ũ��ƫ�ͣ�D������̼���ƾ���ʱ�����������⣬������Na2CO3�������Ũ��ƫ�ߣ�E������ʱ���ӿ̶��ߣ�ʹ��Һ��������Ũ��ƫ�͡�
���㣺���⿼�����ʵij��ӡ���ѧ�������̵ķ�����������������ѧ����ʽ����д��һ�����ʵ���Ũ�ȵ���Һ�����Ƽ���������


 ��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��������ƵĴ��ȿ��õζ������вⶨ��ԭ���ǣ�2S2O32��+ I2 �� S4O62��+ 2I-
38.����100 mL0.0500 mol/L I2��Һ������Ҫ�������� ��ѡ���ţ���
a��100 mL����ƿ b����Ͳ c���ձ� d��������
�ζ��ܱ���ʹ���¶ȣ�20oC; �ζ��ܵ���С�̶�Ϊ mL��
39.ȡ2 .500g�����ʵ�Na2S2O3��5H2O�������50mL��Һ��ÿ��ȡ10.00mL������ƿ���2�ε���Ϊָʾ��������0.0500 mol/L I2��Һ�ζ���ʵ����������(��3�γ�����Ϊ 0.00���յ������ͼ; ���ʲ��μӷ�Ӧ)��
| ��� | 1 | 2 | 3 |
| ����I2��Һ�����/mL | 19.98 | 20.02 | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�Ȼ��Ƴ����ڵ�·�ڱ��������������������ˮ�������������ʡ�ʵ�����ù�ҵ����ʯ����������Al2O3��Fe2O3�����ʣ��Ʊ��Ȼ��Ƶ���Ҫ�������£� ���������գ�
���������գ�
��1������ʹ�õ���������ʵ���Ũ��ԼΪ6.0mol/L������36.5%�����ᣨ�ܶ�Ϊ1.2g/mL������6.0mol/L������100mL������IJ��������в���������Ͳ����ͷ�ιܡ� ����Ҫ��ȡ36.5%������ mL�����ƹ����У���������������ȷ�����в���������Ũ��ƫС���� ��
| A������ҡ�Ⱥ���Һ����ڿ̶��� |
| B������ʱ��������ƿ�Ŀ̶��� |
| C������Һת������ƿ��û��ϴ���ձ��Ͳ���������ת�붨�ݲ��� |
| D�������ˮ�����˿̶��ߣ�ȡ������ˮʹҺ��ǡ�õ��̶��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�����Ƿ�����������FeS2���������ַ������������IJ�������ͼ���£�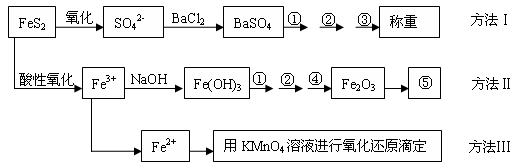
��ش��������⣺
(1)����ͼ�в����١��ڡ��۷ֱ�ָ���Ǣ�_________����__________����________��
�����ܡ����õ�����Ҫ�����ǣ���__________����__________(ÿ����1-2������)��
(2)�ж���Һ��SO42-�����ѳ�����ȫ�ķ�����_________________________________��
(3)ijͬѧ�÷�����ⶨ������FeԪ�صĺ�����ȷ��ȡһ�����Ŀ�ʯ�������������ܽ⡢Ԥ������
| A���ô��п̶ȵ��ձ����Ƴ�100 mL������Һ�� | B������Ͳ��ȡ25.00 mL������Һ�� | C����������ƿ�С� | D��������ˮϴ�ӵζ��ܺ�װ��KMnO4����Һ���øñ���Һ�ζ�����������(E)����Һ��ɵ��Ϻ�ɫʱ��ֹͣ�ζ�����30���ڲ���ɫ��(F)��ȡ������ζ��������ĵ�KMnO4����Һ��������������е�FeԪ�غ�������ָ����ʵ������д����������ı��________________________�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��.��1��ij��ѧ��ȤС����������װ����ѡȡ��Ҫ��װ����ȡ��NH4��2SO4��Һ�����ӵ�˳���ýӿ������ĸ��ʾ���ǣ�a ��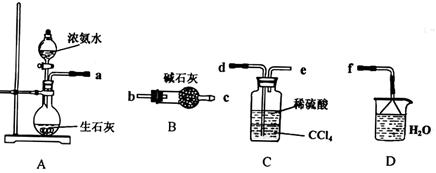
��2����װ��C������Һ����뿪�IJ���������_________��װ��D�������� ��
��.�������ƿ������ڸ��Ƶر�ˮ�ʡ��������ؽ������ӷ�ˮ�������ೱ��Ҳ������Ӧ�������ȡ���ҵ�������������Ƶ���Ҫ�������£�
��֪CaO2��8H2O�ʰ�ɫ������ˮ��I2+2S2O32��= 2I��+S4O62��
��1��������������ȡCaO2��8H2O�Ļ�ѧ����ʽ�� ��
��2�����顰ˮϴ���Ƿ�ϸ�ķ����� ��
��3���ⶨ��Ʒ��CaO2�ĺ�����ʵ�鲽���ǣ�
��һ����ȷ��ȡa g��Ʒ����ƿ�У�������������ˮ������b g KI���壬�ٵ�������2 mol/L��H2SO4��Һ����ַ�Ӧ��
�ڶ�������������ƿ�м��뼸�ε�����Һ��
����������μ���Ũ��Ϊc mol��L��1��Na2S2O3��Һ����Ӧ��ȫ������Na2S2O3��ҺV mL��
���жϴ˵ζ�ʵ��ﵽ�յ�ķ����ǣ� ��
��CaO2����������Ϊ (����ĸ��ʾ)��
��ijͬѧ��һ���͵ڶ����IJ������ܹ淶������������̫����������õ�CaO2��������������
�������Ӱ�족����ƫ�͡���ƫ�ߡ�����ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ʯ����Ҫ�ɷ�ΪCaC2������ΪCaO��CaS���ǹ�ҵ����Ȳ�ij���ԭ�ϣ�ij�о���ѧϰС�������������ַ����ⶨCaC2��������������������и��⡣
����һ����ȡ1.40 g��Ʒ��Բ����ƿ�У��÷�Һ©������������ˮ��ʹ��Ʒ��Ӧ��ȫ������������ɫ
���徭Bϴ����ͨ������װ�ò����Ȳ�������Ϊ��״����448 mL����������Ȳ��ˮ�е��ܽ⣬
��ͬ����
��1����ʯˮ��������Ȳ����Ļ�ѧ����ʽΪ��
��2��������װ���ʵ��Լ��Ĺ��ƿ������˫���������������ɣ��������ʵ���װ�ã�����ͼ�н��䲹����������ע�����ƿ��װ���Լ������ơ�
��3���Ի��ȷ�������������IJ����� ������ţ�
�ټ��װ�õ������ԣ�
�ڻָ������º������µ�����Ͳλ�ã�ʹC��D��Һ����ƽ��
����Aװ�ý�����������һ�����ܣ�ͨ��N2��A��C2H2ȫ�����뵽B��C�У�
�ܶ���ʱ��������Ͳ�ڰ�Һ����͵���ƽ
��4�����������ݿɼ������Ʒ��CaC2����������Ϊ____ ��
����������ȡl.40g��Ʒ����ͼ��ʾʯӢ���У��гּ�����װ��ʡ�ԣ�����a�����ϻ���ͨ���������
������ʯӢ���е���Ʒ����Ӧ��ȫ����ñ���Һ�������ȷ�Ӧǰ������1.80g����Ӧ����ʽΪ��2CaC2+
5O2��2CaO+ 4CO2��
��5����Ӧ��ɺ�ʯӢ������Ʒ����ܱڲ�����ʴ�����û�ѧ����ʽ������ԭ��
��6������ƿ�����Ը��������Һ������Ϊ
��7���ɴ˷�����õ���Ʒ��CaC2�����������ȷ���һ�е�____ �������С������
�ȡ�������ʵ��װ�õĽǶȿ���ԭ��Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����������������ʵ����������������������泥���NH4��2SO4��FeSO4��6H2O����Ʒ����ΪĪ���Σ���һ�ָ��Ρ�һ���������ڿ������ױ������������γɸ��κ�ͱȽ��ȶ�������������һ���������������ˮ�е��ܽ�ȱ��������ÿһ���ε��ܽ�ȶ�С���Ҽ����������Ҵ���������һ���ʿ�����ȡ��������茶��塣�����ε��ܽ�ȣ���λΪg/l00gH2O�����±���
ʵ����Ʒ�� Feм��������̼����3mol/LH2SO4���� NH4��2SO4������ˮ����ˮ�Ҵ���
ʵ�鲽������ͼ��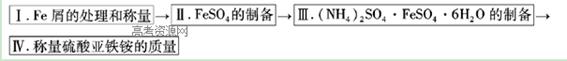
���������ʵ���¼��
��1������I����10% Na2CO3������Һ������м��Ŀ���� ����������ĸ�����м������Ϊm1��
��2���������õ�Feм������ƿ�У���������3mol/LH2SO4��Һ������ˮԡ�м�������������������ʱΪֹ�����������ɣ����������ע�ⰲȫ�������ȹ��ˣ�����������ˮϴ����ƿ����ֽ������Һ��ϴ��Һһ��ת�����������С�����ֽ�ϵĹ��峣�¸������أ���Ϊm2��
��3����ȡһ�������ģ�NH4��2SO4������______ g���ú�m1��m2�Ĵ���ʽ��ʾ��Ҫ����ͬ����ˮ���Ƴ������£�20�棩�ı�����Һ����������뵽����ʵ����������У��������ȣ�Ũ����������ֽᾧ��ĤΪֹ��������ȴ���õ���������淋ľ��壬���˺���____________ϴ�Ӿ��塣
��4����Ʒ���ȵIJⶨ
�ȳ�ȡ��Ʒ1.600g����ˮ�ܽ⣬���100ml��Һ����ȡ25.00mL������Һ����ƿ�У����������ữ��0.0100mol/LKMnO4����Һ���еζ����е�Fe2+���ﵽ�ζ��յ�ʱ���ı�Һ��ƽ��ֵΪ20.00mL������Ʒ�еģ�NH4��2SO4��FeSO4��6H2O����������������ʽ���㣩
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�Ͼ���Ļ������ü������ڽ�Լ��Դ���������ڱ���������ij�о�С��ͬѧ�ԷϾ�п�̸ɵ��Ϊԭ�ϣ����Ͼɵ�غ�п����ת����ZnSO4��7H2O�����̲���ת���ɴ��Ƚϸߵ�MnO2����NH4Cl��ҺӦ���ڻ��������У�ʵ���������£�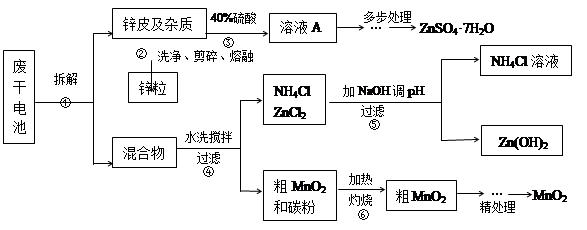
��1�������������õļ�������Ӧѡ ��ѡ�����������������
��2������ҺA�����ĵ�һ���Ǽ��백ˮ����pHΪ9��ʹ���е�Fe3+��Zn2+��������д����ˮ��Fe3+��Ӧ�����ӷ���ʽ ��
��3����������Ϊ�˳�ȥ��Һ�е�Zn2+����֪25��ʱ��
| NH3��H2O��Kb | Zn2+��ȫ������pH | Zn(OH)2���ڼ��pH |
| 1.8��10��5 | 8.9 | ��11 |
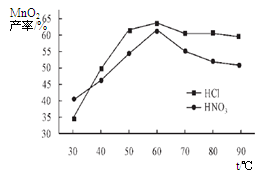
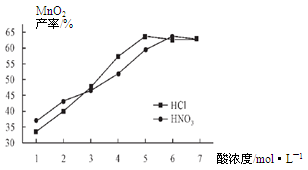
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�÷�Һ©�����Է����һ�������� �� ��
| A���屽��ˮ | B���������������� | C����������Ҵ� | D���Ҵ���ˮ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com