
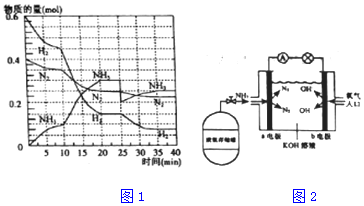
���� ��1�����ݷ�Ӧ����=$\frac{\frac{��n}{V}}{��t}$���㣻
��2������ͼ��֪��ƽ��������Ӧ�����ƶ���10minʱ�������ģ������������ʵ��������ӱ�����ͬ��˵��Ϊʹ�ô�����
��3����ӦN2��g��+3H2��g��=2NH3
a��ƽ��ʱ��Ӧ��2v��H2����=3v��NH3������
b�����������ܶ�һֱ����ʱ��仯���仯��
c�������ڵ���ѹǿ������ʱ��ı仯��˵����������ʵ������䣻
d��N2��H2��NH3�ķ�����֮��Ϊ1��3��2���κ�ʱ�̶�������
e����λʱ������mmolN2��ͬʱ����3mmolH2�������෴����������
f��a mol N��N�����ѵ�ͬʱ����6a mol N-H���γɣ�������ͬ��������Ϊƽ����жϣ�
��4����ѧƽ�ⳣ������������Ũ����֮���뷴Ӧ��Ũ����֮���ıȣ���2��ƽ��ʱNH3������������ڰ����ĺ�����
��5��25���ӣ�NH3�����ʵ���ͻȻ���٣���H2��N2�����ʵ������䣬˵��Ӧ�Ƿ����NH3��
��6�����ݸ�˹��������Ӧ���ʱ䣬Ȼ����ݷ�Ӧ�ų������������ʵ��������ȣ�
��7����ȼ�ϵ���У���������ȼ�ϰ�����ʧ���ӵ�������Ӧ��
�ڸ��ݵ缫��Ӧʽ��������Ӻ�����������Ũ�ȵı仯��ȷ����ҺpH�ı仯���Ӷ����
��� �⣺��1�����ݷ�Ӧ����v��NH3��=$\frac{\frac{��n}{V}}{��t}$=$\frac{\frac{��0.1-0��mol}{2L}}{10min}$=0.005mol/��L��min�����ʴ�Ϊ��0.005mol/��L��min����
��2����ͼ���֪��������ʵ����仯���ӣ���10minʱ�仯�������ģ�20min��ƽ��ʱ����n��N2��=0.025mol��4=0.1mol��
��n��H2��=0.025mol��12=0.3mol����n��NH3��=0.025mol��8=0.2mol�����ʵ����仯֮�ȵ��ڻ�ѧ������֮�ȣ������������ʵ��������ӱ�����ͬ��˵��10min���ܸı��������ʹ�ô����������¶ȣ�Ӧ�÷�Ӧ���ʼ�С������NH3���ʵ������淴Ӧ�������ӵı�����ֻ��ʹ�ô������ϣ�
��ѡa��
�ʴ�Ϊ��a��
��3����ӦN2��g��+3H2��g��=2NH3
a��ƽ��ʱ��Ӧ��2v��H2����=3v��NH3��������a����
b�����������ܶ�һֱ����ʱ��仯���仯����b����
c�������ڵ���ѹǿ������ʱ��ı仯��˵����������ʵ������䣬��Ӧ��ƽ��״̬����c��ȷ��
d��N2��H2��NH3�ķ�����֮��Ϊ1��3��2���κ�ʱ�̶���������d����
e����λʱ������mmolN2��ͬʱ����3mmolH2�������෴������������Ӧ��ƽ��״̬����e��ȷ��
f��a mol N��N�����ѵ�ͬʱ����6a mol N-H���γɣ�������ͬ��������Ϊƽ����жϣ��ʴ���ѡ��c e��
�ʴ�Ϊ��ce��
��4����ѧƽ�ⳣ������������Ũ����֮���뷴Ӧ��Ũ����֮���ıȣ���ͼ���֪��20min��ƽ��ʱ��n��N2��=0.025mol��10=0.25mol��n��H2��=0.025mol��6=0.15mol��n��NH3��=0.025mol��12=0.3mol������������ƽ�ⳣ��K=$\frac{{C}^{2}��N{H}_{3}��}{C��N{\\;}_{2}��{C}^{3}��{H}_{2}��}$=$\frac{��\frac{0.3}{2}��^{2}}{\frac{0.25}{2}����\frac{0.15}{2}��^{3}}$����2��ƽ��ʱNH3���������=$\frac{2.5mol}{2.5mol+2.25mol+0.75mol}$��100%=45.5%��
�ʴ�Ϊ��$\frac{��\frac{0.3}{2}��^{2}}{\frac{0.25}{2}����\frac{0.15}{2}��^{3}}$��45.5%��
��5����25���ӣ�NH3�����ʵ���ͻȻ���٣���H2��N2�����ʵ������䣬˵��Ӧ�Ƿ����NH3����ͼ����Կ���������Ӧ���е�ʱ35-40min�������ʵ������䣬˵����Ӧ�ﵽ�ڶ���ƽ��״̬��ƽ�ⳣ��ֻ���¶�Ӱ�죬�¶Ȳ��䣬ƽ�ⳣ�����䣬���Գ�ȥ0.1mol����
�ʴ�Ϊ�������0.1molNH3��
��6����N2��g��+O2��g���T2NO��g����H=+180.5kJ/mol��
��N2��g��+3H2��g���T2NH3��g����H=-92.4kJ/mol��
��2H2��g��+O2��g���T2H2O��g����H=-483.6kJ/mol��
�ɸ�˹���ɢ١�2-�ڡ�2+�ۡ�3�ã�4NH3��g��+5O2��g���T4NO��g��+6H2O��g����H=905kJ/mol��
��17g ��1mol��������������ȫ����һ�����������ˮ�������ų�������Ϊ$\frac{1}{4}$��905kJ��226.3kJ��
�ʴ�Ϊ��226.3kJ��
��7����ȼ�ϵ���У���������ȼ�ϰ�����ʧ���ӵ�������Ӧ��a�缫ͨ����ǰ�����Ϊ������������ȼ�ϰ���ʧ���ӷ���������Ӧ���缫��ӦʽΪ��2NH3-6e-+6OH-�TN2+6H2O��b�缫Ϊ�������缫��ӦΪ��O2+2H2O+4e-=4OH-��
�ʴ�Ϊ��O2+2H2O+4e-=4OH-��
�ڷ�Ӧһ��ʱ������ڵ�ط�Ӧ����ˮ��4NH3+3O2=2N2+6H2O�����Ե������������Ũ�ȼ�С�������Լ�����pH��С��Ϊά�ּ���Һ��Ũ�Ȳ�����Ҫ��װ���в���KOH��
�ʴ�Ϊ�����ڵ�ط�Ӧ����ˮ��4NH3+3O2=2N2+6H2O�����Ե������������Ũ�ȼ�С�������Լ�����pH��С��Ϊά�ּ���Һ��Ũ�Ȳ�����Ҫ��װ���в���KOH��
���� ���⿼�黯ѧƽ��ļ��㡢ƽ���ƶ���Ӱ�������Լ�ƽ��״̬���жϣ�ע���ͼ��ķ�������Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Na2SO3 | B�� | Na2SO4 | C�� | K2SO4 | D�� | NaHSO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������ | B�� | ������ | C�� | �����ڲ��� | D�� | �ټ�С���ڲ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ʵ��Ŀ�� | ʵ����� |
| A | ����CuSO4��Һ | ������ͭ����������ˮ�У�Ȼ��ϡ�� |
| B | ��ȥ�Թ��ڱڵ����� | ����Ũ��ˮ���ݣ���������ˮϴ�� |
| C | �ᴿ������������Ҵ����Ҵ��е�78.5�棩 | ������ʯ�Һ���������ռ�78.5����� |
| D | ��֤��HClOΪ���� | �ýྻ�IJ�����պȡNaClO��Һ��pH��ֽ�ϣ�����ֽ��ɫ�������ɫ���� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Na2O���ڼ��������� | |
| B�� | Na2CO3���ڱ�¶�ڿ����б�ΪNaHCO3 | |
| C�� | NaOH��Һ�����ڴ��������IJ���ƿ�� | |
| D�� | ��NaHCO3��Һ�еμ�ϡ���ᣬ��ʼʱ������һ��ʱ��������ݲ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NF3���ӵĿռ乹��Ϊ������ | |
| B�� | �����ᣨHN3����һ�����ᣬ�ɲ��ֵ����H+��N3-����N2O��N3-��Ϊ�ȵ����� | |
| C�� | ����������Co3+���γ������磺[Co��N3����NH3��5]SO4���������ܵ���λ��Ϊ8 | |
| D�� | NaN3��KN3�Ľṹ���ƣ���NaN3�ľ����ܴ���KN3�ľ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ƽ��ʱ��X ��ת����Ϊ50% | |
| B�� | ���ն���ƽ�ⳣ��K=10��10mol•L-1��-1 | |
| C�� | 10min��������Z �ķ�Ӧ����0.02mol•L-1•min-1 | |
| D�� | ��Y����ɫ���壬ֻѹ�����������������ϵ��ɫ��dz |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com