
���ǵؿ��к�������Ԫ�ء�
��1����Ԫ�ػ�̬ԭ�Ӻ���δ�ɶԵ�����Ϊ ��.
��2��H2O�����ڵ�O-H�������Ӽ�ķ��»����������ǿ��������Ϊ ��
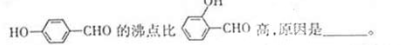
��3�� H+����H2O�γ�H3O+��H3O+��Oԭ�Ӳ��� �ӻ���H3O+��H-O-H���DZ�H2O��H-O-H���Ǵ�ԭ��Ϊ ��
(4)CaO��NaCl�ľ���ͬΪ���������ṹ����֪CaO�����ܶ�Ϊag cm-3,
cm-3,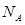 ��ʾ�����ӵ���������CaO�������Ϊ
Cm3��
��ʾ�����ӵ���������CaO�������Ϊ
Cm3��
 ���������������Բ��������ϵ�д�
���������������Բ��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
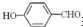 ��
�е��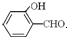 �ߣ�ԭ����
�ߣ�ԭ����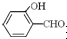 �γɷ������������
�γɷ������������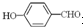 �γɷ��Ӽ���������Ӽ����ʹ���Ӽ�����������
�γɷ��Ӽ���������Ӽ����ʹ���Ӽ����������� �γɷ������������
�γɷ������������ �γɷ��Ӽ���������Ӽ����ʹ���Ӽ�����������
�γɷ��Ӽ���������Ӽ����ʹ���Ӽ�����������| 224 |
| aNA |
| 224 |
| aNA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ������ѧ�߶���ѧ����ĩ���Ի�ѧ��ѡ�ޣ��Ծ� ���ͣ������
̼���γɻ�������������Ԫ�أ���Ԫ�ؿ����γɶ��ֻ�������ǵؿ��к�������Ԫ�ء�
�ش��������⣺��12�֣�ÿ��2�֣�
��1������̼��������������������ģ���Ҫ��Ϊ�˼��� ���ѧʽ�����ŷ�����
��2����̬��ԭ�ӵļ۵����Ų�ʽ��_________ ________��
��3����Ԫ�ػ�̬ԭ�Ӻ���δ�ɶԵ�����Ϊ ����
��4��C��N��O����Ԫ�ص�һ�����ܴӴ�С��˳����____________��
��5����ijԪ��E��ԭ�����������Ų�Ϊ2s22 p1��ijԪ��F��ԭ����Χ�����Ų�Ϊ2s22p2����Ԫ��E��Ԫ��F�ĵ縺�ԴӴ�С˳����_____ _______����Ԫ�ط��ű�ʾ����
Ԫ��F��Ԫ�����ڱ� ������s��p��d��ds��f����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ɽ�� ���ͣ��ʴ���
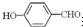
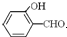
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com