
��ˮռ�����ܴ�ˮ����97%�����Ѻ�ˮ�����ͻ�����������������ȿ��Խ����ˮ��Դȱ�������⣬�ֿ��Գ�����ú�����Դ��
��1����ҵ�����õ�ⱥ��ʳ��ˮ���Ƶ���Ҫ������Ʒ���ֳ�Ϊ���ȼҵ����Ҳ���Ե�������Ȼ����Ƶ��ƺ��������÷�Ӧ����ʽΪ________________________��
��2�����������������һ�������ȼҵ��Ʒ���Ȼ���ѭ������������������������ն�������ķ������÷������������£�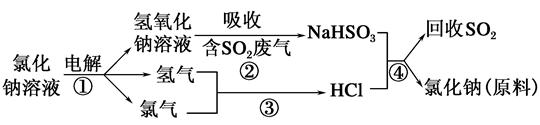
��д����Ӧ�Ļ�ѧ����ʽ��
��__________����__________����__________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��(�۵㣭39�棬�е�356��)�������ء��缫�ȵ���Ҫԭ�ϣ���ʷ�����á����ճ�ɰ������ȡ����Ŀǰ��ҵ���ƴֹ���һ������ͼ���¡�
������������
A�������ճ�ɰ���������е���ת�Ƶķ������Ŀ�ɱ�ʾΪ�� |
| B����ɰ�������Ƽ��ȷ�Ӧʱ��CaSO4Ϊ�������� |
| C��ϴ�Ӵֹ�����5%���������5%������ |
| D����ѹ�����Ŀ���ǽ����ķе㣬��߷���Ч�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�Ӻ�ˮ�п��Ի�õ�ˮ��ʳ�Σ�������ȡþ��������ʡ�
(1)��ˮ�����ķ�����Ҫ��________(��һ��)��
(2)�Ӻ�ˮ����ȡþ����������ͼ��ʾ��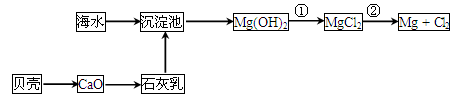
��Ӧ�ٵ����ӷ���ʽΪ________________________________________________��
��Ӧ�ڵĻ�ѧ����ʽΪ________________________________________________��
(3)�Ӻ�ˮ����ȡ�����Ҫ��������Ũ���ĺ�ˮ��ͨ�����������������������÷�Ӧ�����ӷ���ʽΪ________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����Ǹ������Դ���⣬��ˮ��Դ�����þ��зdz������ķ�չǰ������ˮ����Ԫ����Br����ʽ���ڣ���ҵ���ÿ����������Ӻ�ˮ����ȡ��Ĺ�������������ʾ��
(1)����ٷ�Ӧ�����ӷ���ʽΪ_______________________________��
(2)����۷�Ӧ�Ļ�ѧ����ʽΪ_________________________________��
(3)Br��ԭ��������________�������ڱ���λ�ڵ�________���ڡ�________�塣
(4)���������Ĺ����У��¶�Ӧ������80��90�档�¶ȹ�����Ͷ������������������ԭ��________________��
(5)Ϊʲô��ֱ���á���ˮ������Ҫ�á���ˮ�����������ó�Һ�壿
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ȸʯ����Ҫ�ɷ�ΪCu2(OH)2CO3��ijУ��ѧ��ȤС���ͬѧ��ƴӿ�ȸʯ��ұ��ͭ�ķ������£�
�ش��������⣺
(1)�ڷ����Ŀ�ȸʯ�м���ϡ���ᣬ�۲쵽��������_______________��
��Ӧ�����ӷ���ʽ��___________________________________________��
(2)����a���õ��IJ���������____________________________________��
(3)A�Ļ�ѧʽΪ________��������Һ�м���A��Ŀ����______________��
(4)����b����ϴ�Ӻ͵��º�ɣ�������__________________________��
(5)��ͬѧ��Ϊ��������м��ϡ���ᣬ��ͨ������ʵ�鷽����Ҳ�ܴӿ�ȸʯ��ұ��ͭ�������ü�������˵����ͬ������ʵ��ԭ��__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������ۺ����ü������ڽ�Լ��Դ���������ڱ���������ʵ�������÷Ͼɻ�ͭ(Cu��Zn�Ͻ𣬺���������Fe)�Ʊ���������(CuSO4��5H2O)��������ZnO���Ʊ�����ͼ���£� 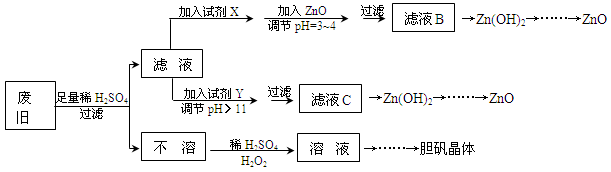
��֪��Zn���������������Al����������������ƣ�pH��11ʱZn(OH)2������NaOH��Һ����[Zn(OH)4]2�����±��г��˼������������������������pH(��ʼ������pH����������Ũ��Ϊ1.0mol��L��1����)��
| | Fe3�� | Fe2�� | Zn2�� |
| ��ʼ������pH | 1.1 | 5.8 | 5.9 |
| ������ȫ��pH | 3.0 | 8.8 | 8.9 |
 2CuI(��ɫ)����I2��I2��2S2O32��
2CuI(��ɫ)����I2��I2��2S2O32�� 2I����S4O62��
2I����S4O62���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ȡ��塢�⡢�ơ�þ���̡�ﮡ��˵�Ԫ���ں����б��������������ú�άȨ�ǹ���ս�ԡ�
��1������Ԫ���ں�ˮ�еĴ�����̬��___________��ѡ�������̬�ڻ���̬�۲�ȷ����
��2�����±仯���ڻ�ѧ�仯����__________________________��
�� �������ѱ䣬�����⣨2H�������˾۱䣬��LiH��Ұ����������ܺ�ˮɹ��
�������ѱ䣬�����⣨2H�������˾۱䣬��LiH��Ұ����������ܺ�ˮɹ��
��3����������ɹ�κ��±ˮ��ȡҺ������ӷ���ʽ______________________________����ʯ�����±ˮ������þԪ�ص����ӷ���ʽ_____________________________________���ɺ����ҿɽ���KI����ij�ֿ��οɽ���KIO3�������������з�Ӧ�����ɵⵥ�ʣ���Ӧ�����ӷ���ʽ__________________________________��
��4����ҵ���Ȼ�����ȡ�����ƵĻ�ѧ����ʽΪ____________________________����ҵ��þ���õ�������Ȼ�þ�������õ����������þ��ԭ����_______________________
��5�����������ֿ����ˣ��̽�������е�һ�֡��̽������Ҫ����MnO2��Fe2O3��һ��������ԼΪm(Mn):m(Fe)=55:448�ĺϽ�֣�����Ԫ���ԣ������п������ҳ����ĥ���������������ĸ�װ�ȡ���ͨ�����ȷ�Ӧ���������ĺϽ�MnO2��Fe2O3��Al��Ͷ�ϱȣ������ʵ���֮�ȣ�ԼΪ_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���������ƣ�Na2S2O5��������ʳƷƯ�������Ʊ������������£�
��֪����Ӧ�����2NaHSO3 Na2S2O5��H2O�ȶಽ��Ӧ��
Na2S2O5��H2O�ȶಽ��Ӧ��
��1��ʵ������ȡ�����Ļ�ѧ����ʽ�� ��
��2����ӦI�Ļ�ѧ����ʽΪ�� ��
��3�������ա�ʱ������Ӧ�Ļ�ѧ����ʽ�� ��
��4����֪Na2S2O5��ϡ���ᷴӦ�ų�SO2�������ӷ���ʽΪ�� ��
��5������ƷX�Ļ�ѧʽ�� ��
��6��Ϊ�˼��ٲ�ƷNa2S2O5�����ʺ���������Ʒ�Ӧ�����������������ʵ���֮��ԼΪ �������Ʒ�к���̼�������������Լ��� �����ţ�
�����Ը������ ��Ʒ����Һ �۳���ʯ��ˮ
�ܱ���̼��������Һ ��NaOH ��ϡ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�����й��������ֵ������������(����)��
| A�����ӱ�Ҫ��Ԫ�أ����Ƹֲĵ���֯�ṹ������ |
| B���ʵ����������еĺ�̼������ȥ���������� |
| C������衢�̡����ȺϽ�Ԫ�ص����ɷֲ���ȥ��ˮ�е��� |
| D����ȥ�����еķǽ���Ԫ�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com