������һ�������Դ��
��1����֪ 2H
2��g��+O
2��g��=2H
2O��g����H=-483.6kJ/mol
2H
2��g��+O
2��g��=2H
2O��l����H=-571.6kJ/mol
H
2��g��+
O
2��g��=H
2O��l����H=-285.8kJ/mol
��������ȼ������
285.8
285.8
kJ/mol��ȼ��2g H
2����ˮ�������ų�������Ϊ
241.8
241.8
kJ��
��2�����ǿ���ͨ������;����ȡ������
��1g̼������ˮ������Ӧ����CO��H
2��������10.94KJ�������˷�Ӧ���Ȼ�ѧ����ʽΪ
C��s��+H2O��g��=CO��g��+H2��g����H=131.28KJ/mol
C��s��+H2O��g��=CO��g��+H2��g����H=131.28KJ/mol
�ڹ�ҵ����������һ����Ҫ��Ӧ�ǣ�CO��g��+H
2O��g��=CO
2��g��+H
2��g������֪��25��ʱ��
H
2��g��+
O
2��g��=H
2O��g����H=-242kJ/molC��ʯī��+O
2��g���TCO
2��g����H=-394kJ/mol
C��ʯī��+
O
2��g���TCO��g��H=-111kJ/mol��25��ʱһ����̼��ˮ��������ת���������Ͷ�����̼��Ӧ�ġ�H=
-41
-41
kJ/mol��



 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
 ������һ�������Դ������ͨ�����ַ����Ƶã�
������һ�������Դ������ͨ�����ַ����Ƶã�

 ������һ�������Դ����������ȡ�봢��������Դ����������о��ȵ㣮
������һ�������Դ����������ȡ�봢��������Դ����������о��ȵ㣮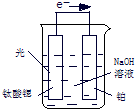 ������һ�������Դ����������ȡ�봢��������Դ����������о��ȵ㣮
������һ�������Դ����������ȡ�봢��������Դ����������о��ȵ㣮