
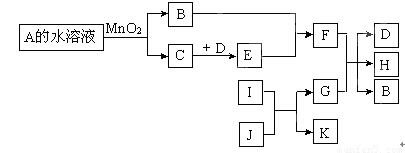
��ͼ��һЩ�������ʡ�������֮���ת����ϵͼ����Щ��Ӧ�еIJ������ʼ���Ӧ��������ȥ��A��B���Ԫ����ͬ���ڳ����¶�����ɫҺ�塣D��E�����Ԫ����ͬ��I��K�����Ԫ����ͬ��I�ڳ���������ɫ�ж����壬J�Ǻ�ɫ��ĩ��G��һ�ֺ�ɫ�������ʡ�
��1��A�Ļ�ѧʽ�� �� ��2��B�ĵ���ʽ�� ��
��3��I��J��Ӧ�Ļ�ѧ����ʽΪ ��
��4����D����ɫ���壬�ҳ�������������Ϊ����ɫ���壬��G��F��Ӧ�����ӷ���ʽΪ
��
��8�֣���1�� H2O2 ��2��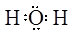 ��3��CuO + CO
��3��CuO + CO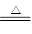 Cu + CO2
Cu + CO2
��4��3 Cu + 8H+ +2NO3-�� 3 Cu2+ +2NO�� + 4 H2O ����2�֣�
��������
���������A��B���Ԫ����ͬ���ڳ����¶�����ɫҺ�壬��˵��Ӧ����ˮ��˫��ˮ��A�ܺͶ������̷�Ӧ������A��˫��ˮ��B��ˮ��C��������I��K�����Ԫ����ͬ��I�ڳ���������ɫ�ж����壬J�Ǻ�ɫ��ĩ��G��һ�ֺ�ɫ�������ʣ����I��CO��J������ͭ��G��ͭ��K��CO2��D��E�����Ԫ����ͬ�����D������NO,E��NO2������F�����ᡣ����DҲ�����Ƕ�������E����������F�����ᡣ
���㣺��������ͼ����й��ж��Լ���ѧ�������д
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣�����ۺ���ǿ���������С���Ҫ�ǿ���ѧ���������仯�������ʺ�ת������Ϥ���ճ̶ȣ�����������ѧ���������������淶����������Ҳ���������ѧ����Ӧ��������ѧϰЧ�ʡ�����������Ҫע����ǻ�ѧ�ƶ�����һ���ۺ��Խ�ǿ�����⣬��Ԫ�ؼ����������ʺ�������������������ѧ�����֪ʶ����������ѧ�Ƽ��ۺϡ��������ɿ���ѧ���Ի�ѧ֪ʶ������̶ȣ�����Ҫ��������ѧ�����ۺϷ���������˼ά���������ͼ��ķ�������ؼ�����Ѱ�ҡ�ͻ�ƿڡ�����ͻ�ƿڡ�����ץ���ء��֣�����������ɫ������״̬��������ζ�����ⷴӦ���������������Ʒ���������;�ȡ�


 ��������ϵ�д�
��������ϵ�д� ����˼ά����ѵ����ʱ��ѧ��ϵ�д�
����˼ά����ѵ����ʱ��ѧ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��12�֣���ͼ��һЩ�������ʡ�������֮���ת����ϵͼ����Щ��Ӧ�еIJ������ʼ���Ӧ��������ȥ��A��B���Ԫ����ͬ���ڳ����¶�����ɫҺ�塣D��E�����Ԫ����ͬ����EΪ����ɫ�����塣I��K�����Ԫ����ͬ��I�ڳ���������ɫ�ж����壬
J�Ǻ���ɫ���壬G��Ŀǰ������ࡢ��;���Ľ������ʡ�
��1��A�Ļ�ѧʽ�� ��
��2��K�ĵ���ʽ�� ��
��3����д��E��B��Ӧ�Ļ�ѧ����ʽ ��
��4����д��G��������Fϡ��Һ��Ӧ�����ӷ���ʽ ��
��5��������G (��ܡ����ܡ�)�ܽ���Ũ��F��Һ�У�ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ�º�����ѧ��һ��ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ������
��12�֣���ͼ��һЩ�������ʡ�������֮���ת����ϵͼ����Щ��Ӧ�еIJ������ʼ���Ӧ��������ȥ��A��B���Ԫ����ͬ���ڳ����¶�����ɫҺ�塣D��E�����Ԫ����ͬ����EΪ����ɫ�����塣I��K�����Ԫ����ͬ��I�ڳ���������ɫ�ж����壬
J�Ǻ���ɫ���壬G��Ŀǰ������ࡢ��;���Ľ������ʡ�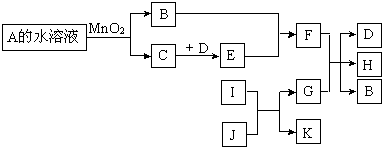
��1��A�Ļ�ѧʽ�� ��
��2��K�ĵ���ʽ�� ��
��3����д��E��B��Ӧ�Ļ�ѧ����ʽ ��
��4����д��G��������Fϡ��Һ��Ӧ�����ӷ���ʽ ��
��5��������G (��ܡ����ܡ�)�ܽ���Ũ��F��Һ�У�ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ������������ѧ��һ��ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ��ƶ���
��ͼ��һЩ�������ʡ�������֮���ת����ϵͼ����Щ��Ӧ�еIJ������ʼ���Ӧ��������ȥ��A��B���Ԫ����ͬ���ڳ����¶�����ɫҺ�塣D��E�����Ԫ����ͬ��I��K�����Ԫ����ͬ��I�ڳ���������ɫ�ж����壬J�Ǻ�ɫ��ĩ��G��һ�ֺ�ɫ�������ʡ�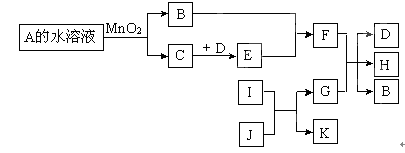
��1��A�Ļ�ѧʽ�� �� ��2��B�ĵ���ʽ�� ��
��3��I��J��Ӧ�Ļ�ѧ����ʽΪ ��
��4����D����ɫ���壬�ҳ�������������Ϊ����ɫ���壬��G��F��Ӧ�����ӷ���ʽΪ
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�콭��ʡ��һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
��12�֣���ͼ��һЩ�������ʡ�������֮���ת����ϵͼ����Щ��Ӧ�еIJ������ʼ���Ӧ��������ȥ��A��B���Ԫ����ͬ���ڳ����¶�����ɫҺ�塣D��E�����Ԫ����ͬ����EΪ����ɫ�����塣I��K�����Ԫ����ͬ��I�ڳ���������ɫ�ж����壬
J�Ǻ���ɫ���壬G��Ŀǰ������ࡢ��;���Ľ������ʡ�
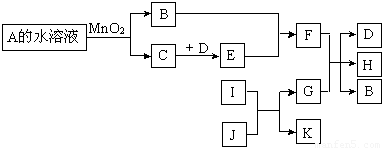
��1��A�Ļ�ѧʽ�� ��
��2��K�ĵ���ʽ�� ��
��3����д��E��B��Ӧ�Ļ�ѧ����ʽ ��
��4����д��G��������Fϡ��Һ��Ӧ�����ӷ���ʽ ��
��5��������G (��ܡ����ܡ�)�ܽ���Ũ��F��Һ�У�ԭ���� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com