
(9��)�ڻ�ƿ�м��롰�ʻ����ʼ��������ӳ��ʻ����������±���500mL���ʻ����ʼ����к��еijɷ֣��Ķ���ش��������⣺
|
�ɷ� |
������g�� |
Ħ��������g ��mol-1�� |
|
���� |
25��00 |
342 |
|
����� |
0��25 |
174 |
|
��˾ƥ�� |
0��17 |
180 |
|
������� |
0��25 |
158 |
|
������ |
0��02 |
170 |
��1�����С��ʻ����ʼ����ijɷ��У����ڷǵ���ʵ���________��
A������ B������� C��������� D��������
��2�����ʻ����ʼ�����K+����˾ƥ���в���K+�������ʵ���Ũ��Ϊ_______ mol/L����ע�⣺ֻҪ����ԭʼ����д����ʽ������Ҫ��������㣩
��3�������������ʻ����ʼ�������������У��ձ���������ƽ��ҩ�ס�________________��______________��_______________�����ں�������д��ȱ���������ƣ�
��4������Һ���ƹ����У����в��������ƽ��û��Ӱ�����___________��
A������ʱ��������ƿ�̶���
B������ƿ��ʹ��ǰδ�����������������ˮ
C������ƿ��ʹ��ǰ�ո�������һ�����ʵ���Ũ�ȵ�NaCl��Һ��δϴ��
D������ҡ�Ⱥ���Һ���������ƿ�Ŀ̶��ߣ���δ���κδ���
��9�֣���1��A��2�֣�
��2��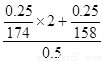 ��2�֣�
��2�֣�
��3������������ͷ�ιܡ�500 mL����ƿ��3�֣�
��4��BD��2�֣�
����������1������������ǵ���ʵ��жϡ�����ˮ������״̬���ܹ�������������ӵĻ������ǵ���ʣ������������¶����ܵ�����������ӵĻ������Ƿǵ���ʣ�����ѡ��BCD��ʧ����ʣ������Ƿǵ���ʣ���ѡA��
��2�����м����ӵķֱ�������غ�����أ����ʵ����ֱ���0.25/174mol��0.25/158mol�����ݻ�ѧʽ��֪��Һ�м����ӵ����ʵ����ǣ�2��0.25/174��0.25/158��mol����˸���c��n/V��֪�������ӵ�Ũ����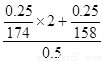
��3������500ml����һ����Ҫ500ml����ƿ���ܽ��ת��ʱ����Ҫ������������ʱ����Ҫ��ͷ�ιܡ�
��4������c��n/V��֪������ʱ��������ƿ�̶��ߣ�������ƿ����Һ�����ƫС��Ũ��ƫ�ߣ�����ƿ����Ҫ���ѡ��B��Ӱ�죻û��ϴ�ӣ���������������ܺ���������Ӧ���ɰ�ɫ�����Ȼ�����Ӱ��ʵ������ѡ��D��δ���κδ�������ȷ�ģ�ʵ���ѡBD��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�갲��ʡ�Ϸ�һ�и�һ��ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ�ʵ����
(9��)�ڻ�ƿ�м��롰�ʻ����ʼ��������ӳ��ʻ����������±���500mL���ʻ����ʼ����к��еijɷ֣��Ķ���ش��������⣺
| �ɷ� | ������g�� | Ħ��������g ��mol-1�� |
| ���� | 25��00 | 342 |
| ����� | 0��25 | 174 |
| ��˾ƥ�� | 0��17 | 180 |
| ������� | 0��25 | 158 |
| ������ | 0��02 | 170 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com