
ʵ��������NaOH��������1.0mol/L��NaOH��Һ480mL��
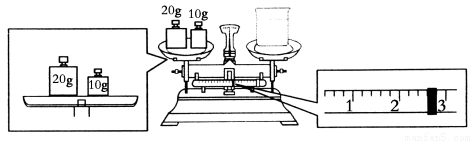
��1������ʱ������ʹ�õIJ���������________��________��________��________��
��2��Ҫ��ɱ�ʵ���ͬѧӦ�Ƴ�NaOH________g��
��3��ijͬѧ������NaOH��������������������ƽ�����ձ�����������ƽƽ����״̬��ͼ���ձ���ʵ������Ϊ________g��
��4��ʹ������ƿǰ������е�һ��������________��
��5�������ƹ����У���������������ȷ�ģ����в������������ƫ�ߵ���________��
��û��ϴ���ձ��Ͳ����� ��ת����Һʱ������������������ƿ����
������ƿ�����������������ˮ �ܶ���ʱ���ӿ̶���
��δ��ȴ�����¾ͽ���Һת�Ƶ�����ƿ������
���ݺ�����ƿ������ҡ�ȣ����ú�Һ����ڿ̶��ߣ��ټ�ˮ���̶��ߡ�
��1���ձ�����������500 mL����ƿ����ͷ�ι�
��2��20.0 ��3��27.4 ��4���������ƿ�Ƿ�©ˮ ��5���ܢ�
��������
�����������1��û��480mL����ƿ��ѡ��500mL����ƿ�����������м��㡢�������ܽ⡢��Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣬�ò��������裬�����ܽ⣬�ָ����º�ת�Ƶ�500mL����ƿ�У����ò�����������ϴ��2-3�Σ�����ϴ��Һ��������ƿ�У�����ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ�������Ҫ��������������ƽ���ձ���500ml������ƿ����ͷ�ιܡ�ҩ�ף���Ϊ���ձ���500ml������ƿ������������ͷ�ιܣ�����2�����������Ƶ�����Ϊm=0.5L��1mol?L-1��40g/mol=20.0g����Ϊ��20.0����3��������ƽ���øܸ�ԭ�����죬ԭ�����������룬������ͼ��ʾ��30=m+2.6���ó�m=27.4g, ��Ϊ��27.4����4����Ϊ�����ߵ�ҡ�ȣ���������ƿ��ʹ��ǰ�������Ƿ�©ˮ����Ϊ���������ƿ�Ƿ�©ˮ����5����û��ϴ���ձ��Ͳ����������������������٣�������ҺŨ��ƫ�ͣ���ת����Һʱ������������������ƿ���棬���������������٣�������ҺŨ��ƫ�ͣ�������ƿ�����������������ˮ������ҺŨ����Ӱ�죻�ܶ���ʱ���ӿ̶��ߣ�������Һ���ƫС��������ҺŨ��ƫ�ߣ���δ��ȴ�����¾ͽ���Һת�Ƶ�����ƿ�����ݣ��¶Ȼָ����ºᵼ����Һ���ƫС����ҺŨ��ƫ�ߣ����ݺ�����ƿ������ҡ�ȣ����ú�Һ����ڿ̶��ߣ��ټ�ˮ���̶��ߣ�������Һ���ƫ��������ҺŨ��ƫ�ͣ�ѡ���ܢݣ�
���㣺����һ�����ʵ���Ũ����Һ�����ơ�


 ��ѧ����ͬ����ϰϵ�д�
��ѧ����ͬ����ϰϵ�д� ��ǰ�κ�ͬ����ϰϵ�д�
��ǰ�κ�ͬ����ϰϵ�д� ����С��ҵϵ�д�
����С��ҵϵ�д� �Ƹ�С״Ԫ����������ϰ��ϵ�д�
�Ƹ�С״Ԫ����������ϰ��ϵ�д� �ɹ�ѵ���ƻ�ϵ�д�
�ɹ�ѵ���ƻ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�����ʡ��һ�ϵڶ��ο��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
����������ȷ����( )��
A��NaCl��Ħ��������58.5 g
B��1 mol NaCl��������58.5 g��mol-1
C��58.5 g NaCl����Na����Cl����1mol
D����1 mol NaCl����Һ��Լ����6.02��1023��Cl��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�����ʡ��һ��ѧ�����л�ѧ�Ծ��������棩 ���ͣ�ѡ����
ͬ��ͬѹ�£�1���X2������3���Y2���廯������2������廯�����û�����Ļ�ѧʽΪ
A��XY B��XY3 C��X3Y D��X2Y3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�����ʡ�����и߶�10���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
A��B��C��D��������֮��ɷ�����Ӧ��aA + bB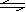 cC + d D���÷�Ӧ��һ�������½���ƽ�⣬�ı����������㹻����ʱ�����ϵ���������и��ֱ仯�������ܱ�����ƽ��һ��������ת�Ƶ���( )
cC + d D���÷�Ӧ��һ�������½���ƽ�⣬�ı����������㹻����ʱ�����ϵ���������и��ֱ仯�������ܱ�����ƽ��һ��������ת�Ƶ���( )
A����������ܶ�������
B�������ϵ��ѹǿ������
C�����淴Ӧ��������ȣ��Ҷ���ԭƽ��״̬������������
D��A�����ڻ�����еĺ���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�����ʡ�����и߶�10���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
��֪���ȼ��a g��Ȳ��C2H2������ʱ����2 mol������̼�����Һ̬ˮ�����ų�����b kJ������Ȳȼ���ȵ��Ȼ�ѧ����ʽ��ȷ����( )
A��2C2H2��g����5O2��g����4CO2��g����2H2O��l�� ��H����2b kJ / mol
B��C2H2��g����5/2O2��g����2CO2��g����H2O��l�� ��H����b kJ / mol
C��2C2H2��g����5O2��g����4CO2��g����2H2O��l�� ��H����4b kJ / mol
D��2C2H2��g����5O2��g����4CO2��g����2H2O��l�� ��H��b kJ / mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�����ʡ�����и�һ10���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
�ڱ�״���£��� L�����к��е���ԭ�Ӹ���Ϊ
L�����к��е���ԭ�Ӹ���Ϊ �����ӵ������ɱ�ʾΪ�� ��
�����ӵ������ɱ�ʾΪ�� ��
A��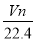 B��
B��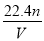 C��
C��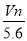 D��
D��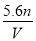
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�����ʡ�����и�һ10���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
��״�����Т�6.72L���� ��3.01��1023���Ȼ������ ��13.6g���� ��0.2mol NH3�����ж�����������Ĺ�ϵ��С�����ʾ����ȷ����( )
A���������<��<��<�� B���ܶȣ���<��<��<��
C����������<��<��<�� D����ԭ��������<��<��<��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�����ʡ�����и߶���ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
����ͼ��ʾ����H1=��393.5 kJ?mol-1����H2=��395.4 kJ?mol-1������˵�����ʾʽ��ȷ���� �� ��
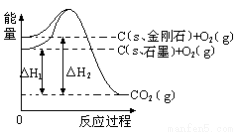
A��C��s��ʯī��== C��s�����ʯ����H= +1.9 kJ?mol-1
B��ʯī�ͽ��ʯ��ת���������仯
C�����ʯ���ȶ���ǿ��ʯī
D��1 molʯī���ܼ��ܱ�1 mol���ʯ���ܼ���С1.9 kJ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�����ʡ��һ10���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
��VL���� ��
��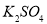 �Ļ����Һ�ֳ����ȷ�,һ�ݼ��뺬a mol NaOH����Һ,ǡ��ʹþ������ȫ����Ϊ������þ;��һ�ݼ��뺬b mol BaCl2����Һ,ǡ��ʹ�����������ȫ����Ϊ���ᱵ����ԭ�����Һ�м����ӵ�Ũ��Ϊ( )
�Ļ����Һ�ֳ����ȷ�,һ�ݼ��뺬a mol NaOH����Һ,ǡ��ʹþ������ȫ����Ϊ������þ;��һ�ݼ��뺬b mol BaCl2����Һ,ǡ��ʹ�����������ȫ����Ϊ���ᱵ����ԭ�����Һ�м����ӵ�Ũ��Ϊ( )
A�� 
 B��
B�� 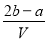
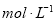
C�� 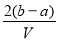
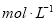 D��
D�� 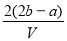

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com