
ij���᳧�����Ŀ�����Ҫ��Ⱦ��Ϊ���������Ϊ�˱����������ۺ����ã��ɲ��ð�-���������շ����˷����м����պͰ������������ŵ㣬�����չ����������£�
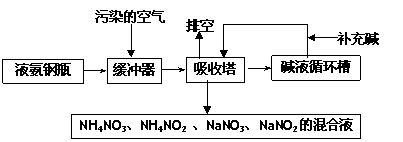 |
��1���ſ����ʵ���Ҫ�ɷ�Ϊ____________________________��
��2��������������ǰ��һ����������Ŀ����________________________________��
��3�����������ų��Ļ��Һ��;֮һΪ________________ ��
��������Ҫ��Ⱦ��Ϊ���������͵���������о���Ա�����ͬʱ���������ж�������͵���������ķ���������ת��Ϊ��������ᣬ�����������£�
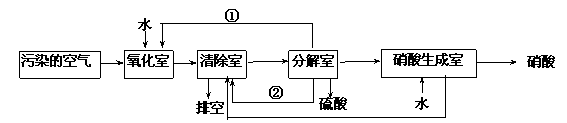
�����з����ķ�Ӧ���£�
a.�����ң�NO2(g) + SO2(g) + H2O (l)=H2SO4 (l)+ NO(g)��H=a kJ��mol-1
b.����ң�NO (g)+ NO2 (g)= N2O3(g) ��H= b kJ��mol-1
N2O3 (g)+ 2H2SO4(l) = 2NOHSO4 (s)+ H2O(l) ��H=c kJ��mol-1
c.�ֽ��ң�4NOHSO4 (s)+ O2 (g)+ 2H2O (l)= 4H2SO4 (l)+ 4NO2(g) ��H= dkJ��mol-1
�ش��������⣺
��4���ٺ͢ڷֱ�Ϊ��д��ѧʽ��______________��_____________��
��5��д��SO2�� O2 �� H2O��Ӧ����H2SO4���Ȼ�ѧ����ʽ��
____________________________________________________________��
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ���� |
| 140�� |
| ���� |
| 140�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� |
| 140�� |
| ���� |
| 140�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ�ձ�����2012�������һ�ε��в��Ի�ѧ���� ���ͣ�058
ij���᳧�����Ŀ�����Ҫ��Ⱦ��Ϊ���������Ϊ�˱����������ۺ����ã��ɲ��ð������������շ����˷����м����պͰ������������ŵ㣬�����չ����������£�
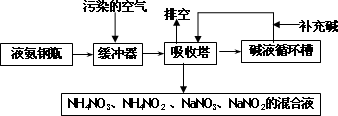
(1)�ſ����ʵ���Ҫ�ɷ�Ϊ________��
(2)������������ǰ��һ����������Ŀ����________��
(3)���������ų��Ļ��Һ��;֮һΪ________��
��������Ҫ��Ⱦ��Ϊ���������͵���������о���Ա�����ͬʱ���������ж�������͵���������ķ���������ת��Ϊ��������ᣬ�����������£�
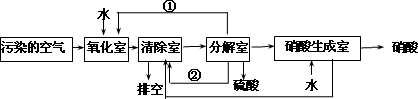
�����з����ķ�Ӧ���£�
a�������ң�NO2(g)��SO2(g)��H2O(l)![]() H2SO4(l)��NO(g)����H��akJ��mol��1
H2SO4(l)��NO(g)����H��akJ��mol��1
b������ң�NO(g)��NO2(g)��N2O3(g)����H��b kJ��mol��1
N2O3(g)��2H2SO4(l)![]() 2NOHSO4(s)��H2O(l)����H��c kJ��mol��1
2NOHSO4(s)��H2O(l)����H��c kJ��mol��1
c���ֽ��ң�4NOHSO4(s)��O2(g)��2H2O(l)![]() 4H2SO4(l)��4NO2(g)����H��d kJ��mol��1
4H2SO4(l)��4NO2(g)����H��d kJ��mol��1
�ش��������⣺
(4)�ٺ͢ڷֱ�Ϊ(д��ѧʽ)________��________��
(5)д��SO2��O2��H2O��Ӧ����H2SO4���Ȼ�ѧ����ʽ��________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com