��2013?���϶�ģ����Ԫ�صĻ����������������������Ź㷺��Ӧ�ã�
��1��400�棬1.01��10
5Pa�£��ݻ�Ϊ1.0L���ܱ������г���0.5molSO
2����g����0.3molO
2 ��g��������2SO
2��g��+O
2��g��?2SO
3��g����H=-198kJ?mol
-1��Ӧ��n��SO
3����n��O
2����ʱ��仯�Ĺ�ϵ��ͼ1��ʾ����Ӧ��ƽ�ⳣ��K=
160
160
��0��10min����SO
2����ʾ��ƽ����Ӧ����
0.04mol/��L?min��
0.04mol/��L?min��
������ͼ1����Ϣ���ж�������������ȷ����
AC
AC
������ţ���
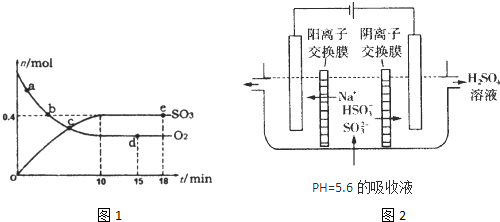
A��a��ʱ�̵�����Ӧ���ʱ�b��ʱ�̵Ĵ�
B��c��ʱ�̷�Ӧ�ﵽƽ��״̬
C��d���e��ʱ�̵�c��O
2����ͬ
D����500�棬1.01��10
5Pa�£���Ӧ�ﵽƽ��ʱ��n�� SO
3����ͼ��e��ʱ�̵�ֵ��
��2����NaOH��Һ���չ�ҵ�����е�SO
2��������Һʧȥ��������ʱ��25��ʱ�����Һ��pH=5.6����Һ��Na
+��H
+��SHO
3-��SO
32-���ӵ�Ũ���ɴ�С��˳����
c��Na+����c��HSO3-����c��H+����c��SO32-��
c��Na+����c��HSO3-����c��H+����c��SO32-��
��3����ͨ����ⷨʹ��2���е�����Һ������ѭ�����ã��缫��Ϊʯī�缫�����乤��ʾ��ͼ��ͼ2��
HSO3-�������ҷ�Ӧ�ĵ缫��ӦʽΪ
HSO3-+H2O-2e-=SO42-+3H+
HSO3-+H2O-2e-=SO42-+3H+
�������ҵIJ���
H2��NaOH
H2��NaOH
��




