
R��X+R��OH![]() R��O��R��+HX
R��O��R��+HX
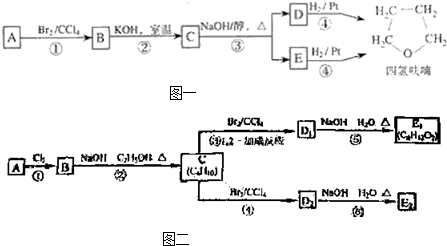
������A�������IJ���Ӧ�ɵõ������ܼ�����ૣ���Ӧ��ͼ��ͼ1-4-27

ͼ1-4-27
��ش��������⣺
(1)1 mol A��1 mol H2��һ��������ǡ�÷�Ӧ�����ɱ���һԪ��Y��Y��̼Ԫ�ص���������ԼΪ65%����Y�ķ���ʽΪ_____________________________________________��
A���������������ŵ�������____________________________________________��
A�Ľṹ��ʽΪ________________________________________________________��
(2)�ڢ٢ڲ���Ӧ���ͷֱ�Ϊ��__________________��__________________��
(3)������B���еĻ�ѧ����(��д��ĸ����)��_________��
a.�ɷ���������Ӧ
b.ǿ���ǿ�������¾��ɷ�����ȥ��Ӧ
c.�ɷ���������Ӧ
d.�������¿ɷ����Ӿ۷�Ӧ
(4)д��C��D��E�Ľṹ��ʽ��
C__________________��D��E__________________
(5)д��������C��NaOHˮ��Һ��Ӧ�Ļ�ѧ����ʽ��
____________________________________________________________________
(6)д���������״���������ͬ���칹��Ľṹ��ʽ��___________________________��
�������ɱ���һԪ����ͨʽCnH2n+2O�ɵã�![]() ��100%=65%���n=4��y�ķ���ʽΪC4H10O������1 mol A����1 mol H2ǡ����ȫ��Ӧ��˵��A�ķ��ӽṹ�к���һ��C=C��һ��
��100%=65%���n=4��y�ķ���ʽΪC4H10O������1 mol A����1 mol H2ǡ����ȫ��Ӧ��˵��A�ķ��ӽṹ�к���һ��C=C��һ�� ��������A�������CCl4��Һ��Ӧ��˵��A��һ������C=C����A�������Ĺ�����Ϊ̼̼˫�����ǻ���������Ľṹ��ʽ���ƿɵ�D(E)�Ľṹ��ʽΪ
��������A�������CCl4��Һ��Ӧ��˵��A��һ������C=C����A�������Ĺ�����Ϊ̼̼˫�����ǻ���������Ľṹ��ʽ���ƿɵ�D(E)�Ľṹ��ʽΪ![]() ���������Ŀ������Ϣ��֪C�Ľṹ��ʽΪ
���������Ŀ������Ϣ��֪C�Ľṹ��ʽΪ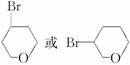 ����C����A
����C����A![]() B
B![]() C�õ��ģ��ʡ�Br�롪OH��������ͬһ̼�ϣ�Ҳ���������ĸ�̼�����ˣ�˵��C�Ľṹ��ʽΪ
C�õ��ģ��ʡ�Br�롪OH��������ͬһ̼�ϣ�Ҳ���������ĸ�̼�����ˣ�˵��C�Ľṹ��ʽΪ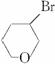 ��B�Ľṹ��ʽΪHO��CH2��CH2
��B�Ľṹ��ʽΪHO��CH2��CH2![]() CH2Br����B�ɷ���a��b��c���ַ�Ӧ������ૺ��л�״�ṹ��������״��ͬ���칹���бض�����̼̼˫���������������ͬ���칹��ֱ�ΪCH2=CHOCH2CH3��
CH2Br����B�ɷ���a��b��c���ַ�Ӧ������ૺ��л�״�ṹ��������״��ͬ���칹���бض�����̼̼˫���������������ͬ���칹��ֱ�ΪCH2=CHOCH2CH3��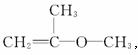 ,
,
CH2=CHCH2��O��CH3��CH3CH=CHO��CH3��
�𰸣�(1)C4H10O �ǻ� ̼̼˫�� CH2=CHCH2CH2��OH
(2)�ӳ� ȡ��
(3)abc
(4)C��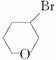 D��E��
D��E��
(5)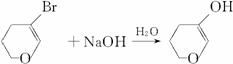 +NaBr��
+NaBr��
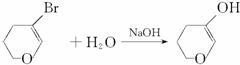 +HBr
+HBr
(6)CH2=CHOCH2CH3 ![]() CH2=CHCH2OCH3 CH3CH=CHOCH3
CH2=CHCH2OCH3 CH3CH=CHOCH3



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| KOH/���� |
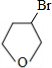





 +NaOH
+NaOH| ˮ |
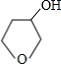 +NaBr
+NaBr +NaOH
+NaOH| ˮ |
 +NaBr
+NaBr| �� |
| �� |
| �� |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
![]()
������A�������IJ���Ӧ�ɵõ������ܼ�����ૣ���Ӧ��ͼ���£�

������������⣺
(1)1 mol A��1 mol H2��һ��������ǡ�÷�Ӧ�����ɱ���һԪ��Y��Y��̼Ԫ�ص���������ԼΪ65%����Y�ķ���ʽΪ____________��A���������������ŵ�������____________��A�Ľṹ��ʽΪ____________��
(2)�ڢ٢ڲ���Ӧ���ͷֱ�Ϊ����______________________����______________________��
(3)������B���еĻ�ѧ����(��д��ĸ����)��____________��
a.�ɷ���������Ӧ b.ǿ���ǿ�������¾��ɷ�����ȥ��Ӧ
c.�ɷ���������Ӧ d.�������¿ɷ����Ӿ۷�Ӧ
(4)д��C��D��E�Ľṹ��ʽ��C_______________��D��E_______________��
(5)д��������C��NaOHˮ��Һ��Ӧ�Ļ�ѧ����ʽ��_____________________________��
(6)д���������״���������ͬ���칹��Ľṹ��ʽ��__________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������£�±�����봼��Ӧ������(R��O��R��):
![]()
������A�������IJ���Ӧ�ɵõ������ܼ�����ૣ���Ӧ��ͼ���£�

������������⣺
(1)1 mol A��1 mol H2��һ��������ǡ�÷�Ӧ�����ɱ���һԪ��Y��Y��̼Ԫ�ص���������Ϊ65%����Y�ķ���ʽΪ________________��A���������������ŵ�������_______________��A�Ľṹ��ʽΪ_______________��
(2)�ڢ٢ڲ���Ӧ���ͷֱ�Ϊ��_______________����_______________��
(3)������B���еĻ�ѧ����(��д��ĸ����)��_______________��
a.�ɷ���������Ӧ
b.ǿ���ǿ�������¾��ɷ�����ȥ��Ӧ
c.�ɷ���������Ӧ
d.�������¿ɷ����Ӿ۷�Ӧ
(4)д��C��D��E�Ľṹ��ʽ��
C_____________��D��E______________________________��
(5)д��������C��NaOHˮ��Һ��Ӧ�Ļ�ѧ����ʽ��_______________________________��
(6)д���������״���������ͬ���칹��Ľṹ��ʽ��____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꼪��ʡ������ʮһ�и߶���ѧ���ڳ����Ի�ѧ�Ծ����������� ���ͣ������
��12�֣���֪������£�±�����봼��Ӧ������(R1��O--R2)��
������A�������IJ���Ӧ�ɵõ������ܼ�����ૣ���Ӧ��ͼ����
��ش��������⣺
(1)1molA��1molH2��һ��������ǡ�÷�Ӧ�����ɱ���һԪ��Y��Y��̼Ԫ�ص���������ԼΪ65%����Y�ķ���ʽΪ ��A�����������ŵ������� A�Ľṹ��ʽ
(2)�ڢ٢۲���Ӧ�����Ǣ� ��
(3)��֪E�ĺ˴Ź��������г��������,д��C����E�Ļ�ѧ����ʽ
(4)A�кܶ��칹��,�����ܺ�������ͭ��Ӧ���ɺ�ɫ�������� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com