
�����ᣨH3PO3���Ƕ�Ԫ�ᣬH3PO3��Һ���ڵ���ƽ�⣺H3PO3 H+ + H2PO3����������������NaOH��Һ��Ӧ������Na2HPO3��
H+ + H2PO3����������������NaOH��Һ��Ӧ������Na2HPO3��
��1����д��������������NaOH��Һ��Ӧ�����ӷ���ʽ_____________________________________��
��ij�¶��£�0.1000 mol��L��1��H3PO3��ҺpH�Ķ���Ϊ1.6������ʱ��Һ��c (H+) = 2.5��10��2 mol��L��1����OH��֮���������ӵ�Ũ����С�����˳���� �����¶���H3PO3����ƽ���ƽ�ⳣ��K= ����H3PO3�ڶ���������Բ��ƣ����������λ��Ч���֣�
����H3PO3��Һ�еμ�NaOH��Һ�����ԣ�������Һ��c��Na+��_______ c��H2PO3-��+ 2c��HPO32-�����>���� ��<�� ��=������
��2�����������ǿ��ԭ�ԣ���ʹ��ˮ��ɫ���÷�Ӧ�Ļ�ѧ����ʽ_______________________��
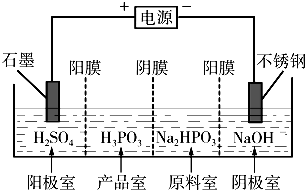
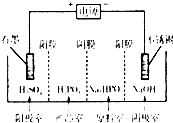
��3�����Na2HPO3��ҺҲ�ɵõ������ᣬװ��ʾ��ͼ���£�
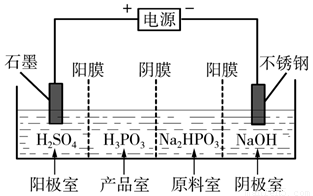
˵������Ĥֻ����������ͨ������Ĥֻ����������ͨ����
�������ĵ缫��ӦʽΪ_____________________________��
�ڲ�Ʒ���з�Ӧ�����ӷ���ʽΪ_____________________��
��14�֣���1�� �� H3PO3+OH����H2PO3��+H2O ��2�֣�
��c��HPO32-��< c��H2PO3-��< c��H+�� ��2�֣� 8.3��10��3mol/L ��2�֣� �� ����2�֣�
��2��H3PO3 + I2 +H2O = 2HI + H3PO4 ��2�֣� ��3���� 2H+ + 2e����H2�� ��2�֣�
��HPO32��+ 2H+��H3PO3 ��2�֣���HPO32��+ H+ ��H2PO3�� ��H2PO3��+ H+��H3PO3����1�֣�
��������
�����������1�����������Ƕ�Ԫ�ᣬ������������������Ʒ�Ӧ����NaH2PO3��H2O�����Ը÷�Ӧ����ʽΪH3PO3+OH����H2PO3��+H2O��
��0.1000mol•L-1��H3PO3��ҺpH�Ķ���Ϊ1.6��������Ũ��С��������Ũ�ȣ������������Ƕ�Ԫ���ᣬ��ˮ��Һ�зֲ����룬�ҵ�һ������̶ȴ��ڵڶ��������������ж������������ɣ�����������Ũ������������Ũ�ȴ�С˳����c��HPO32-��< c��H2PO3-��< c��H+����
H3PO3
 H+ +
H2PO3��
H+ +
H2PO3��
��ʼʱ������Ũ�ȣ�mol•L��1�� 0.10 0 0
��Ӧ�ĸ����ʵ�Ũ�ȣ�mol•L��1��2.5��10��2 2.5��10��2 2.5��10��2
ƽ��ʱ�����ʵ�Ũ�ȣ�mol•L��1��0.10��2.5��10��2 2.5��10��2 2.5��10��2
K��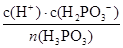 ��
��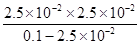 ��8.3��10��3mol/L
��8.3��10��3mol/L
����Һ�����ԣ���c��H+����c��OH-��������Ϊ��Һ�ʵ����ԣ���c��Na+��+C��H+����C��OH-��+c��H2PO3-��+2c��HPO32-��������C��H+��=C��OH-��������c��Na+����c��H2PO3-��+2c��HPO32-����
��2�������ǿ�����ԣ����������ǿ��ԭ�ԣ�����������͵��ܷ���������ԭ��Ӧ�������������ᣬ��Ӧ�Ļ�ѧ����ʽΪH3PO3 + I2 +H2O = 2HI + H3PO4 ��
��3���ٵ����������õ����ӣ�������ԭ��Ӧ�������������������ӵõ��ӷ�����ԭ��Ӧ���缫��ӦʽΪ2H+ + 2e����H2����
�ڲ�Ʒ����HPO32-�������ӽ�����������ᣬ��˷�Ӧ���ӷ���ʽΪHPO32��+ 2H+��H3PO3��
���㣺������ʵĵ��롢����ƽ�ⳣ���ļ��㡢��Һ��������Ũ�ȴ�С�Ƚϡ�������ԭ��Ӧ����ʽ����д�Լ��绯ѧԭ����Ӧ�õ�


 ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2013?������ģ�������ᣨH3PO3���Ƕ�Ԫ�ᣬH3PO3��Һ���ڵ���ƽ�⣺H3PO3?H++H2PO3-��������������NaOH��Һ��Ӧ������Na2HPO3��
��2013?������ģ�������ᣨH3PO3���Ƕ�Ԫ�ᣬH3PO3��Һ���ڵ���ƽ�⣺H3PO3?H++H2PO3-��������������NaOH��Һ��Ӧ������Na2HPO3���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ������ | ���� | ���� | ������ | |
| ������ | Cl-OH |  |
 |
 |
| ���ǻ� ��ԭ���� |
0 | 1 | 2 | 3 |
| ���� | ���� | ��ǿ�� | ǿ�� | ��ǿ�� |




�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��֪��Cu��OH��2�Ƕ�Ԫ��������ᣨH3PO3���Ƕ�Ԫ���ᣬ��NaOH��Һ��Ӧ������Na2HPO3��
��֪��Cu��OH��2�Ƕ�Ԫ��������ᣨH3PO3���Ƕ�Ԫ���ᣬ��NaOH��Һ��Ӧ������Na2HPO3���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com