
�����±��е��ĸ��Ȼ�ѧ����ʽ���ж������ͱ����ȼ���ȷֱ��ǣ� ��
| ���϶�һ�š�������ȼ�� | Һ�⣨H2�� | ��2H2(g)+O2(g)=2H2O(l)����H=-571.6kJ��mol-1 ��2H2(l)+O2(l)=2H2O(g)����H=-482.6kJ��mol-1 |
| �������˻ᡰ���ơ����ȼ�� | ���飨C3H8�� | ��C3H8(l)+5O2(g)=3CO2(g)+4H2O(g)�� ��H=-2013.8kJ��mol-1 �� C3H8(g)+5O2(g)=3CO2(g)+4H2O(l)�� ��H=-2221.5kJ��mol-1 |
A.571.6 kJ��mol-1��2221.5kJ��mol-1 B.241.3 kJ��mol-1��2013.8 kJ��mol-1
C.285.8 kJ��mol-1��2013.8 kJ��mol-1 D.285.8 kJ��mol-1��2221.5 kJ��mol-1

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
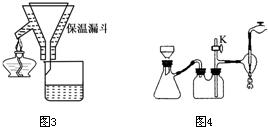 �������A��B��С�⣬�ֱ��Ӧ�ڡ����ʽṹ�����ʡ��͡�ʵ�黯ѧ������ѡ��ģ������ݣ���ѡ������һ�⣬������Ӧ�ʹ������������������ⶼ������A�����֣�
�������A��B��С�⣬�ֱ��Ӧ�ڡ����ʽṹ�����ʡ��͡�ʵ�黯ѧ������ѡ��ģ������ݣ���ѡ������һ�⣬������Ӧ�ʹ������������������ⶼ������A�����֣�
| ������/kJ?mol-1 | I1 | I2 |
| ͭ | 746 | 1958 |
| п | 906 | 1733 |
| NaNO3 | KNO3 | NaCl | KCl | |
| 10�� | 80.5 | 20.9 | 35.7 | 31.0 |
| 100�� | 175 | 246 | 39.1 | 56.6 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com