��2009?����һģ��������þ������ҩ��ҵ��������Ҫ����ɫ��ȼ����
I������θ�����ҩ��Stmoache����Ч�ɷ�ΪMg��OH��
2��
��1����ҩ������θ�ᣨ��Ҫ�ɷ�Ϊ���ᣩ����֢ʱ��Ӧ�����ӷ���ʽ��
Mg��OH��2+2H+=Mg2++2H2O
Mg��OH��2+2H+=Mg2++2H2O
��
����֪��
Mg ��s��+2H
2O��g��=Mg��OH��
2��s��+H
2��g����H
1=-441kJ?mol
-1H
2O��g��=H
2��g��+
O
2��g����H
2=+242kJ?mol
-1Mg��s��+
O
2��g��=MgO��s����H
3=-602kJ?mol
-1��2��������þ�ֽ���Ȼ�ѧ����ʽ��
Mg��OH��2��s��=MgO��s��+H2O��g������H=+81kJ?mol-1
Mg��OH��2��s��=MgO��s��+H2O��g������H=+81kJ?mol-1
��
��3��������þ������Ϊ��ȼ����ԭ��
������þ�ֽ�ų���������
������þ�ֽ�ų���������
����дһ�����ɣ�
��ij��������ˮ���Ȼ�þ�ʹ�ʯ����ȡ��������þ�������������������ʣ�ͨ����ͼ���̽����ᴿ���ƣ������ȼ��������þ��
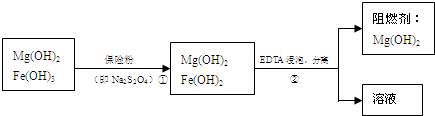
��4���������������������
����
����
��
��5��������еķ�Ӧ���£�6Fe��OH��
3+S
2O
42-+2OH
-=6Fe��OH��
2+2SO
42-+4H
2O��ÿ����0.1mol���շۣ�Na
2S
2O
4��ʱ��ת�Ƶ��ӵ���Ŀ��
0.6
0.6
N
A��
��6����֪EDTAֻ������Һ�е�Fe
2+��Ӧ����������ˮ�����ʣ�����Mg��0H��
2��Ӧ����ȻFe��OH��
2������ˮ���������������EDTA�ļ��룬�����ܹ���Fe��OH��
2��ȥ����ô��ȸߵ�Mg��OH��
2����ӳ����ܽ�ƽ��ĽǶȼ��Խ��ͣ�
��
Fe��OH��2����Һ�д�������ƽ�⣺Fe��OH��2?Fe2++2OH-������EDTA�ļ��룬EDTA�����Fe2+����ʹƽ�������ƶ����ܽ�
Fe��OH��2����Һ�д�������ƽ�⣺Fe��OH��2?Fe2++2OH-������EDTA�ļ��룬EDTA�����Fe2+����ʹƽ�������ƶ����ܽ�
��
����Ϊ�о���ͬ�����ᴿ���������Ƶ���ȼ���Ĵ��ȴӶ�ȷ������ᴿ������ij�о�С���ȡ������������4���������Ƶõ���ȼ�����к������IJⶨ��������£�
| ������ȼ�������� |
��ȼ�������� |
| ��� |
�ᴿ��ϵ�¶�T/�� |
����EDTA������g�� |
���뱣�շ�������g�� |
W��Fe��/��10-4g�� |
| 1 |
40 |
0.05 |
0.05 |
7.63 |
| 2 |
40 |
0.05 |
0.10 |
6.83 |
| 3 |
60 |
0.05 |
0.10 |
6.83 |
| 4 |
60 |
0.10 |
0.10 |
6.51 |
��7�������������������������ϱ����ݣ���ȡ�ߴ�����ȼ�����������
C
C
������ĸ��
��40���60���EDTA����Ϊ0.05g ��EDTA����Ϊ0.10g �ݱ��շ�����Ϊ0.05g �ޱ��շ�����Ϊ0.10g
A���٢ۢ�B���ڢܢ�C���٢ܢ�D���ڢۢ�



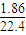 ��0.083mol��
��0.083mol��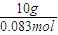 ��120.5g/mol��
��120.5g/mol��

 ����5��2���ϵ�д�
����5��2���ϵ�д�
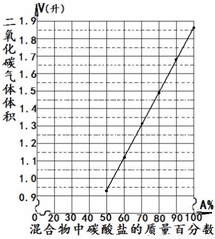
 ��2009?�Ͼ���ģ����ҵ�Ͽ����úϳ�����CO��H2�Ļ�����壩�����״����練Ӧ�ܣ�����֪��
��2009?�Ͼ���ģ����ҵ�Ͽ����úϳ�����CO��H2�Ļ�����壩�����״����練Ӧ�ܣ�����֪�� �±���һ���10.0�ˣ�������̼���Σ�M2CO3������Ʒ��������ϡ���ᷴӦ����������̼�����������ѻ���ɱ�״���µ����������Ʒ��̼���ε������ٷ��������ݣ�
�±���һ���10.0�ˣ�������̼���Σ�M2CO3������Ʒ��������ϡ���ᷴӦ����������̼�����������ѻ���ɱ�״���µ����������Ʒ��̼���ε������ٷ��������ݣ�
 �±���һ���10.0�ˣ�������̼���Σ�M2CO3������Ʒ��������ϡ���ᷴӦ����������̼�����������ѻ���ɱ�״���µ����������Ʒ��̼���ε������ٷ��������ݣ�
�±���һ���10.0�ˣ�������̼���Σ�M2CO3������Ʒ��������ϡ���ᷴӦ����������̼�����������ѻ���ɱ�״���µ����������Ʒ��̼���ε������ٷ��������ݣ�