
| |||||||||||||||||||||||||
(1) |
���ˮ�����������������ģ��������������ȡ�������ǽϾ�������Դ�ɳ������õ����ⷽ���� |
(2) |
�����𰸣�4.36��1 �������������Ȼ�ѧ����ʽ��֪����ͬ������������̼��ȫȼ��ʱ�ų�������֮�ȣ� ����(285.8 kJ��mol��1�� |
(3) |
�����𰸣�PdH0.8 �����������������֪��1 cm3�ٷۿ�����896 cm3�������� ����n(Pd)��n(H)�� �������⻯��Ļ�ѧʽΪPdH0.8�� |


 ���ƽ̸�������ѡ����ĩ���100��ϵ�д�
���ƽ̸�������ѡ����ĩ���100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A.���ˮ B.п��ϡ���ᷴӦ
C.��⺣ˮ D.��ʯ�͡���Ȼ��Ϊԭ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1��Ϊ����Ч��չ��������Դ�����ȱ����Ƶ����۵����������пɹ������ֽϾ�������Դ�ɳ������õ��������ķ�����____________��
A.���ˮ B.п��ϡ���ᷴӦ
C.��⺣ˮ D.��ʯ�͡���Ȼ��Ϊԭ��
��2������ȼ��ʱ������С������������֪�Ȼ�ѧ����ʽΪ��
C��g��+O2(g)====CO2(g)����H= -393.5 kJ��mol-1
H2(g)+![]() O2(g) ====H2O(l)����H=-285.8 kJ��mol-1
O2(g) ====H2O(l)����H=-285.8 kJ��mol-1
��ͨ������˵����������������̼ȼ��ʱ���������ı���________��
��3������Դ�п���ʵ����Դ�����棬Ҳ�п���ʵ�־��á���Ч�����͡��о��������ɽ������⻯��ֳƼ���⻯����������⻯���У���ԭ������ڽ����ľ����϶֮�䣬����ɲ��̶���ͨ���Ƿǻ�ѧ�����ģ��磺LaH2.76��TiH1.73��CeH2.69��ZrH1.98��PrH2.85��TaH0.78����֪��״���£�1������ٷ۴�Լ������896������������ٷ۵��ܶ�Ϊ10.64 g��cm-3�����ԭ������Ϊ106.4������д���٣�Pd�����⻯��Ļ�ѧʽ��___________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ����12��������⻯ѧ�Ծ��������棩 ���ͣ������
��15�֣���ѧ����Ϊ��������һ�ָ�Ч������Ⱦ��������Դ����20����������������Ϊδ���Ķ���ȼ������Դ���о������Ѹ�ٷ�չ��
��1��Ϊ����Ч��չ��������Դ�����ȱ����Ƶ����۵����������пɹ������ֽϾ�������Դ�ɳ������õ��������ķ����� ����ѡ����ĸ��
A�����ˮ B��п��ϡ���ᷴӦ
C����⺣ˮ D���ֽ���Ȼ��
��2����ˮ�ֽ��������������仯����ͼ��ʾ����ʾʹ�ô��������� ���÷�ӦΪ (���Ȼ�������)��Ӧ

��3��1g��������ȫȼ������Һ̬ˮ�ͷų�142.9kJ������д������ȫȼ�յ��Ȼ�ѧ����ʽ�� ��
��4������������CO�ϳɶ����ѵ�������Ӧ���£�
�� 2H2(g) + CO(g)  CH3OH(g)����H ����90.8 kJ��mol��1
CH3OH(g)����H ����90.8 kJ��mol��1
�� 2CH3OH(g)  CH3OCH3(g) + H2O(g)����H����23.5 kJ��mol��1
CH3OCH3(g) + H2O(g)����H����23.5 kJ��mol��1
�� CO(g) + H2O(g)  CO2(g) + H2(g)����H����41.3 kJ��mol��1
CO2(g) + H2(g)����H����41.3 kJ��mol��1
�ܷ�Ӧ��3H2(g)
+ 3CO(g)  CH3OCH3(g) + CO2
(g)�Ħ�H��
CH3OCH3(g) + CO2
(g)�Ħ�H��
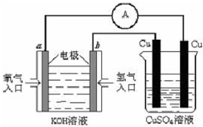
��5������ȼ�ϵ������ת���ʸߣ����й����ķ�չǰ������������ȼ�ϵ�ؽ�����ͼ��ʾʵ�飺

������ȼ�ϵ���У������ĵ缫��ӦʽΪ ��
����ͼװ���У�ijһͭ�缫����������3.2g���� a �������ĵ�O2�ڱ�״���µ����Ϊ
L��
��6����������Ѱ����ʵĴ����͵缫���ϣ���N2��H2Ϊ�缫��Ӧ���HCl��NH4ClΪ�������Һ��ȡ����ȼ�ϵ�ء��������缫����ʽ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com