
����Ч�ļ���ȼ�ϵ�ز��ò�Ϊ�缫���ϣ����缫�Ϸֱ�ͨ��CH4��O2�������ΪKOH��Һ��ij�о�С�齫��������ȼ�ϵ�ش�������Ϊ��Դ�����б����Ȼ�����Һ�����м��η�̪�����ʵ�飬��ͼ��ʾ��
�ش��������⣺
��1������ȼ�ϵ�������������ĵ缫��Ӧ�ֱ�Ϊ________________________ ��________________________��
��2���պ�K���غ�a��b�缫�Ͼ����������������a�缫�ϵõ�����________����������________�����a����b������������ֺ�ɫ������Ȼ�����Һ���ܻ�ѧ����ʽΪ________________________����a��b�����IJ������Ӧ�ɵõ���84������Һ����Ч�ɷ�NaClO����������¿��á�84������Һ����SO2����Ӧ�����ӷ���ʽΪ________________________��
��3����ÿ����ؼ���ͨ����Ϊ1 L����״�������ҷ�Ӧ��ȫ��������������ܲ������������Ϊ________L����״������
 �߽�������ϵ�д�
�߽�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ��ʾˮ�����Թ�����һö���������������۲죺
(1)�����������⣬�������ĸ�ʴ����________��ʴ��
(2)���Թ���Һ����������ԭ��Һ��________�ԣ�����________��ʴ���缫��ӦʽΪ��������____________________��������____________________��
(3)���Թ���Һ���½�����ԭ��Һ��________�ԣ�����________��ʴ���缫��ӦʽΪ��������____________________��������____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ⱻ��Ϊ������Ԫ�ء�����ѧ�����ز����ɷ��ε�ȱ������KI��KIO3���Ⱥ����ڼӵ����С�
(1)��ҵ�Ͽ���ͨ����м������KI���乤���������£�
�ٷ�Ӧ�����������Ļ�������û���������Ԫ�����Ԫ�ص�������Ϊ21��127�����������̼���ʱ����Ӧ��Ļ�ѧ����ʽΪ____________________��
�ڲ���A����__________________���ñ�ˮϴ�ӵ�Ŀ����__________________��
(2)KIO3����ͨ��H2O2����I2���Ƶ�HIO3��Ȼ������KOH�к͵ķ�������������
�����ʱ����KIO3��ʳ�γ��ڳ���ǰ���룬��ԭ����_____________________________
�����Ƶ�1 284 kg KIO3���壬������������������������Ϊ30%��˫��ˮ________ kg��
��KIO3����ͨ����ͼ��ʾԭ�������Ʊ������ʱ�ܷ�Ӧ�����ӷ���ʽΪ____________����������Һ����ı仯�������������������pH����ǰ���________(ѡ���������С�����䡱)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ö��Ե缫���NaCl��NaHCO3�Ļ����Һ�������Һ��pH�仯��ͼ��ʾ��
��1����0��t1ʱ���ڣ������缫�ϵĵ缫��ӦʽΪ��
������________________________________________________________________________��
������________________________________________________________________________��
��2�������ӷ���ʽ��ʾ0��t1ʱ���ڣ���ҺpH���߱Ƚϻ�����ԭ��____________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪Ǧ���صĹ���ԭ��ΪPb+PbO2+2H2SO4  2PbSO4+2H2O��������ͼװ�ý��е��(���Һ����)����õ�Ǧ������ת��0.4 mol ����ʱ���缫����������11.2 g����ش��������⡣
2PbSO4+2H2O��������ͼװ�ý��е��(���Һ����)����õ�Ǧ������ת��0.4 mol ����ʱ���缫����������11.2 g����ش��������⡣
(1)A��Ǧ���ص� ����Ǧ����������ӦʽΪ ���ŵ�����е��Һ���ܶ� (���С�����������䡱)��
(2)Ag�缫�ĵ缫��Ӧʽ�� ���õ缫�ĵ缫���ﹲ g��
(3)Cu�缫�ĵ缫��Ӧʽ�� ��CuSO4��Һ��Ũ�� (���С�����������䡱)��
(4)��ͼ��ʾ�����й�����ij����(������x)��ʱ��ı仯���ߣ���x��ʾ ��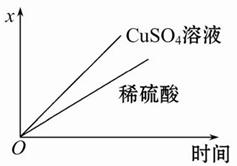
a.��U�ι��в�������������
b.��U�ι������������ļ�����
c.��U�������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1����Al��NiO��OH��Ϊ�缫��NaOH��ҺΪ���Һ���һ�����͵�أ��ŵ�ʱNiO��OH��ת��ΪNi��OH��2�õ�ط�Ӧ�Ļ�ѧ����ʽ��________��
��2����ѧ�ڻ�������������ʮ����Ҫ�����ã���������ѧ���͵绯ѧ���ⷨ����������ˮ�������ε���Ⱦ���绯ѧ����NO��ԭ����ͼ��ʾ��
�ٵ�Դ����Ϊ__________����A��B����������ӦʽΪ________________________��
������������ת����2 mol���ӣ���Ĥ������Һ�������仯���m������m����Ϊ__________�ˡ�
��3������֮����ת�������ʳ��ˮ�Ʊ�Cl2�ǽ�����ת��Ϊ��ѧ�ܣ���ԭ��ؿɽ���ѧ��ת��Ϊ���ܡ�����������͵�ԭ��أ�̽��������ת��Ч�ʡ���ѡ���ϣ�ZnSO4��aq����FeSO4��aq����CuSO4��aq����ͭƬ����Ƭ��пƬ�͵��ߡ�
�����ԭ��ؼ�װ��ʾ��ͼ����ͼ����������Ӧ��ע��Ҫ����ͬһ�ձ��У��缫����Һ����ͬ�Ľ���Ԫ�ء�
����ͭƬΪ�缫֮һ��CuSO4��aq��Ϊ�������Һ��ֻ��һ���ձ�����װԭ����ң�����һ��ʱ��ɹ۲쵽����__________��
�ۼ�������ԭ��ؿɸ���Ч�ؽ���ѧ��ת��Ϊ���ܵ���________����ԭ����_________________________________________________________________��
�ܸ�����������������������ԭ����Ϊ�����������Һ����Ƭ�ĸ�ʴ���������IJ�����Ӧѡ__________��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ�����ø�����(FeO Cr2O3)ұ�����Ĺ�����������:
Cr2O3)ұ�����Ĺ�����������:
��1��ʵ���ұ��ո������ѡ�õ�װ����__________������ţ���
��2��������л�ѧ����ʽ���ں�������д���ʵĻ�ѧʽ������������4CrO42����6S��7H2O��4Cr(OH)3����3S2O32����_____________��
��3������I����������ϴ�ӣ�����ʵ������ϴ�ӳ����IJ�����__________________��
��4��Cr(OH)3��Al(OH)3���ƣ�Ҳ�������������д��Cr(OH)3����Ũ����������Һ�����ӷ���ʽ________________________________��
��5����Cr2O3ұ��Cr�Ļ�ѧ����ʽΪ____________________________��
��6��Ŀǰ��һ�ֲ����Ը�����(Na2CrO4)Ϊԭ�ϣ��õ绯ѧ���Ʊ��ظ�����(Na2Cr2O7)��ʵ��װ������ͼ��ʾ����֪��2CrO42����2H�� Cr2O72����H2O����
Cr2O72����H2O����
��д�������ĵ缫��ӦʽΪ________________________________________��
�ڵ��һ��ʱ������������Һ��Na�����ʵ�����a mol��Ϊb mol�����ʱ�����Ƶ�ת����Ϊ___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ�ϲ��õ�һ����ˮ���������ǣ�������ˮ��pH��5.0��6.0֮�䣬ͨ���������Fe(OH)3������Fe(OH)3�������ԣ�������������������������о���ˮ�����á�ij����С���ô˷�������ˮ�����װ��ʾ��ͼ����ͼ��ʾ��
��1��ʵ��ʱ����ˮ������Ũ�Ƚ�С�����������ϲ��ʹ�������γɸ�������ʱ��Ӧ����ˮ�м���������________��
| A��BaSO4 | B��CH3CH2OH | C��Na2SO4 | D��NaOH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������ӵ��������Դ�Ŀ�����ռ����Ҫ��λ�����������ܻ����綯�����Ķ�����ء���������ﮣ�LiFePO4������������ӵ�ص���ѡ�缫���ϣ������Ʊ��������£�
����һ����̼��ﮡ�����������(CH3COO) Fe�ݡ��������藺�һ��������ϡ������ĥ����800�����ҡ����������Χ�������Ƶþ�̬��������ﮣ�ͬʱ���ɵ����ἰ����������������ݳ���
Fe�ݡ��������藺�һ��������ϡ������ĥ����800�����ҡ����������Χ�������Ƶþ�̬��������ﮣ�ͬʱ���ɵ����ἰ����������������ݳ���
����������һ��Ũ�ȵ��������李��Ȼ�﮻����Һ��Ϊ���Һ��������Ϊ������ʯīΪ���������������������﮳��������������ˡ�ϴ�ӡ������800�����ҡ����������Χ�������Ƶþ�̬��������ﮡ�
������ӵ���У���Ҫһ���л��ۺ�����Ϊ������֮�������Ǩ�ƵĽ��ʣ����л��ۺ���ĵ���֮һ����M��ʾ���Ľṹ��ʽ���£�
��ش��������⣺
��1���������ַ����Ʊ���������﮵Ĺ��̶������ڶ��������Χ�н��С���ԭ������������������������������������������������������������������������������
��2���ڷ���һ�������ķ�Ӧ�У� ��������������ﮡ������⣬ �����������������������������������������������������������ѧʽ�����ɡ�
��3���ڷ������У�����������������﮵ĵ缫��ӦʽΪ��������������������������������������������������������������������������
��4��д��M����������������Һ��Ӧ�Ļ�ѧ����ʽ����������������������������������������������������������������������������
��5����֪������ӵ���ڳ������У������������������������������õ�طŵ�ʱ�����ĵ缫��ӦʽΪ��������������������������������������������������������������������������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com