
| ���� | X | Y | Z |
| ��ʼ���ʵ���/mol | 0��1 | 0��3 | 0 |
| ƽ�����ʵ���/mol | 0��05 | 0��2 | 0��1 |
| A�� | ��Ӧ�ﵽƽ��ʱ��X��ת����Ϊ50% | |
| B�� | ��Ӧ�ɱ�ʾΪX+2Y?2Z | |
| C�� | ����ʼʱX��Y��Z�����ʵ����ֱ�Ϊ0.1 mol��0.4mol��0.2mol����ƽ��ʱ��Z���������һ������ | |
| D�� | ����ʼʱX��Y��Z�����ʵ����ֱ�Ϊ0.05 mol��0.15mol��0.1mol����ƽ��ʱ��X��ת����һ����ԭͶ����� |
���� A������ת����=$\frac{���ʵ����仯��}{��ʼ���ʵ���}$��100%���㣻
B���������ʵ����仯��֮�ȵ��ڻ�ѧ������֮�ȣ��ݴ��жϻ�ѧ����ʽ��
C����ЧΪ��ԭƽ��Ļ�����ѹǿ����һ�������ƽ���ƶ��жϣ�
D����ЧΪ��ԭƽ��Ļ����Ͻ�Y��С0.5mol��ƽ�����淴Ӧ�����ƶ����ݴ˷�����
��� �⣺A����Ӧ�ﵽƽ��ʱ��X��ת����Ϊ��$\frac{0.1mol-0.05mol}{0.1mol}$��100%=50%����A��ȷ��
B����ѧ������֮�ȵ������ʵ����仯��֮�ȣ����ݱ��е����ݿ�֪��X��Y�����ʵ�����С������Ϊ��Ӧ�Z�����ʵ������ӣ�Ϊ�������n��X��������n��Y������n��Z����=0.05��0.1��0.1=1��2��2����Ӧ�ķ���ʽΪX+2Y?2Z����B��ȷ��
C�����ݷ�ӦX+2Y?2Z����0.2molZȫ��ת��ΪX��Y����X��Y����ʼ���ʵ���Ϊ0.2mol��0.6mol����ЧΪ��ԭƽ��Ļ�����Ũ������һ����ƽ��������Ӧ�����ƶ���ƽ��ʱZ�������������C��ȷ��
D�����ݷ�ӦX+2Y?2Z����0.1molZȫ��ת��ΪX��Y����X��Y����ʼ���ʵ���Ϊ0.1mol��0.25mol����ЧΪ��ԭƽ��Ļ����Ͻ�Y��С0.5mol��ƽ�����淴Ӧ�����ƶ�����X��ת�����½�����D����
��ѡD��
���� ���⿼�黯ѧƽ����㡢��ѧƽ��Ӱ�����ء���Чƽ��ȣ����ؿ���ѧ����֪ʶ�����⣬�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ͬ������H2��Cl2��H2�ķ�����һ����Cl2�� | |
| B�� | 0.5mol���������0.5g | |
| C�� | Ħ�����������������Ӷ��ٵ�һ�������� | |
| D�� | 0.1mol H2SO4������ԭ�����ľ�ȷֵΪ1.204��1023 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
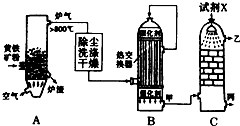 �ϳɰ���ҵ�����Ṥҵ�����Ṥҵ�ǻ�ѧ��ҵ����Ҫ��ɲ��֣���ش��������⣺
�ϳɰ���ҵ�����Ṥҵ�����Ṥҵ�ǻ�ѧ��ҵ����Ҫ��ɲ��֣���ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 CO2��CO�ǹ�ҵ�ŷŵĶԻ�������Ӱ��ķ�����
CO2��CO�ǹ�ҵ�ŷŵĶԻ�������Ӱ��ķ�����| ���� | CH3OH | CH3OCH3 | H2O |
| Ũ��/��mol•L-1�� | 0.01 | 0.2 | 0.2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �۳� | B�� | �����ЧӦ | C�� | �����˶� | D�� | ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | SO42-? | B�� | Na+ | C�� | Cl- | D�� | CH3COO- |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com