
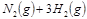
 2NH3(g)���ڹ�ҵ�����еĴ������ã��������˿ڵļ�����������ʳ������ҲΪ���������ṩ���㹻��ԭ�ϡ���Ҫ��ش��������⣺
2NH3(g)���ڹ�ҵ�����еĴ������ã��������˿ڵļ�����������ʳ������ҲΪ���������ṩ���㹻��ԭ�ϡ���Ҫ��ش��������⣺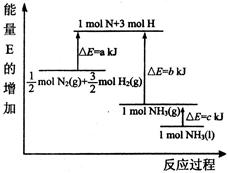
 ��1��
��1��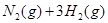
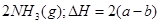
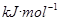 ��2�֣�
��2�֣�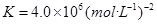 ��2�֣�
��2�֣�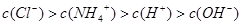 ��2�֣�
��2�֣�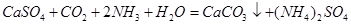 ��2�֣������ڣ�1�֣�
��2�֣������ڣ�1�֣�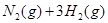

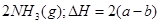
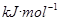
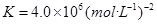 ��
��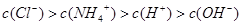 ��
��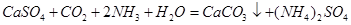 ��
��

 �ۺ��Բ�ϵ�д�
�ۺ��Բ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| | ���� | | ���� | | ���� |
| H��H | 436 | Cl��Cl | 243 | H��Cl | 432 |
| S��S | 255 | H��S | 339 | C��F | 427 |
| C��Cl | 330 | C��I | 218 | H��F | 565 |
| C��O | 347 | H��O | 464 | Si��Si | 176 |
| Si��O | 460 | O=O | 497 | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����֪2H2(g)��O2(g)��2H2O(l)����H����483.6 kJ��mol��1����������ȼ����Ϊ483.6 kJ��mol��1 |
| B����֪C(ʯī��s)��C(���ʯ��s)����H��0������ʯ��ʯī�ȶ� |
| C����֪2C(s)��2O2(g)��2CO2(g)����H1��2C(s)��O2(g)��2CO(g)����H2����H1����H2 |
| D����֪Ni(CO)4(s)��Ni(s)��4CO(g)����H��+Q kJ��mol��1����Ni(s)��4CO(g)��Ni(CO)4(s)����H����Q kJ��mol��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����6a+5d-4c-12b�� kJ��mol-1 |
| B����4c +12b -6a-5d�� kJ��mol-1 |
| C����4c +12b -4a-5d�� kJ��mol-1 |
| D����4a+5d-4c -12b�� kJ��mol-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ��
�� ��ʵ����N��N������Ϊ167kJ��mol��1��NO2�е���˫����ƽ������Ϊ466 kJ��mol��1��N2O4�е���˫����ƽ������Ϊ438.5 kJ��mol��1��
��ʵ����N��N������Ϊ167kJ��mol��1��NO2�е���˫����ƽ������Ϊ466 kJ��mol��1��N2O4�е���˫����ƽ������Ϊ438.5 kJ��mol��1�� N2O4 (g)�����¶�ΪT1��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ��ʾ������˵����ȷ����( )
N2O4 (g)�����¶�ΪT1��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ��ʾ������˵����ȷ����( )
| ʱ�䣨s�� | 0 | 20 | 40 | 60 | 80 |
| n(NO2)/mol | 0.40 | n1 | 0.26 | n3 | n4 |
| n(N2O4)/mol | 0.00 | 0.05 | n2 | 0.08 | 0.08 |
 N2O4(g)�÷�Ӧ��ƽ�ⳣ��K��ֵΪ ����100��ʱ��ijʱ�̲��c(NO2)=1.00mol/L,c(N2O4)=0.20mol/L�����ʱ�̵�v�� ���� v�����>������<����=�����������¶Ⱥ�Ӧ2NO2
N2O4(g)�÷�Ӧ��ƽ�ⳣ��K��ֵΪ ����100��ʱ��ijʱ�̲��c(NO2)=1.00mol/L,c(N2O4)=0.20mol/L�����ʱ�̵�v�� ���� v�����>������<����=�����������¶Ⱥ�Ӧ2NO2 N2O4��ƽ�ⳣ��K�� �����������С�����䡱����
N2O4��ƽ�ⳣ��K�� �����������С�����䡱�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
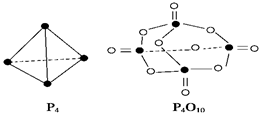
 O2��g����
O2��g���� P4O10��s�� ��H����738.5kJ��mol��1
P4O10��s�� ��H����738.5kJ��mol��1 �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ��������̼������,�������з�����������ʵ�ֽ��ܼ��š��������õȡ�
��������̼������,�������з�����������ʵ�ֽ��ܼ��š��������õȡ�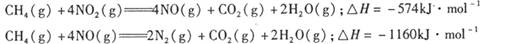
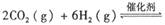
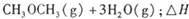 ����֪��һ��ѹǿ��,�÷�Ӧ���¶ȵ����߶�CO2��ת���ʽ��͡���÷�Ӧ��
����֪��һ��ѹǿ��,�÷�Ӧ���¶ȵ����߶�CO2��ת���ʽ��͡���÷�Ӧ��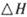 ________ 0(� >���� <�����������Լ��ѡ�����������������ҺΪԭ�ϣ�ʯīΪ�缫����ȼ�ϵ��,��õ���и����ĵ缫��Ӧʽ��________________________________,�ŵ��������Һ��PH________ (���������С�����䡱����
________ 0(� >���� <�����������Լ��ѡ�����������������ҺΪԭ�ϣ�ʯīΪ�缫����ȼ�ϵ��,��õ���и����ĵ缫��Ӧʽ��________________________________,�ŵ��������Һ��PH________ (���������С�����䡱�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����824.4kJ��mol�� 1 | B����627.6kJ��mol��1 |
| C����744.7kJ��mol��1 | D����169.4kJ��mol��1 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com