
Fe2O3���й㷺����;��
��ͬѧ�Ķ��й����ϵ�֪���ڸ���������FeCO3 ���Եõ�Fe2O3��Ϊ�˽�һ����֤�˽��ۣ�����������ʵ�飺
| ʵ�鲽�� | ʵ����� |
| �� | ȡһ��������FeCO3�������������У������������������ټ��ᣬ��ȴ�����¡� |
| �� | ȡ����ʵ�鲽������ù������һ�ྻ���Թ��У���������ϡ�����ܽ⡣ |
| �� | ��ʵ�鲽���������Һ�еμ�KSCN��Һ����Һ��졣 |
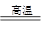 2Fe2O3+4CO2
2Fe2O3+4CO2 ���100��1�ž�ϵ�д�
���100��1�ž�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
һλѧ����������������Ӧ��IJ������̽����
(1)�������
����1������ΪFeO��
����2��__________________��
(2)��������
��ѧ��ͨ���������ϵ�֪�������������������У��������������ȶ�������������ȶ��������¼��ױ�����������������(��ɫ�ɺ�ɫ��ɺ�ɫ)��
ͨ���������Ͽ��Եó��ij�������Ϊ_________________________________��
(3)����ʵ��
��ѧ�����������װ�ý���������������Ӧ��ʵ�顣�����������ʵ�鲽�貹��������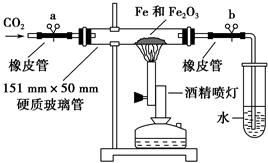
����ͼװ�����Ӻ�����(�ݲ�װ��ҩƷ)��___________________ ____��
�ڳ�ȡ1 g��ԭ�����ۺ�5 g��������ĩ����Ͼ��Ⱥ�ƽ̯�ڲ������в���
���ɿ��������ɼУ�_______________________________________________��
���ɼ��ϵ��ɼ�a������ʼ����ҩƷ��
�ܴ�Լ4�������ң���ɫ��ĩȫ����ڣ��ټ��ϵ��ɼ�b��Ȼ��ֹͣ���ȣ��ȵ���������ȴ�����£�������ɫ��ĩ��
(4)���ṩ����ҩƷ����֤ʵ��õ��ĺ�ɫ��ĩ�ijɷ֡�������ϡ���ᡢKSCN��Һ������KMnO4��Һ���Թܡ���ͷ�ιܡ�
| ʵ�鲽�� | Ԥ������ͽ��� |
| | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ͭ���ʼ��仯�����Ӧ�÷�Χ�ܹ㡣���к��Ȼ��������ʵ��Ȼ�ͭ����(CuCl2��2H2O),Ϊ��ȡ������CuCl2��2H2O�����Ƚ����Ƴ�ˮ��Һ��Ȼ������ͼ��������ᴿ��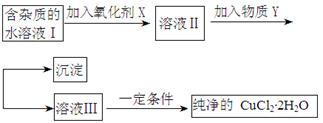
��֪Cu2+��Fe3+��Fe2+���������↑ʼ�����ͳ�����ȫʱ��pH���±���
| | Fe3+ | Fe2+ | Cu2+ |
| �������↑ʼ����ʱ��pH | 1.9 | 7.0 | 4.7 |
| �������������ȫʱ��pH | 3.2 | 9.0 | 6.7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
þ�ڸ�������O2��N2��CO2�����Է�����Ӧ���Խ���������⡣
��1����ҵͨ�����õ�������Ȼ�þұ������þ����������þΪԭ�ϵ������� ��
��2����ȼ�ŵ�þ������ʢ��CO2�ļ���ƿ�У�þ������ȼ�գ�����ҫ�۵İ⣬ƿ�ڱ����к�ɫ�������ɣ���ѧ����ʽΪ ��
��3���ɽ��ʯ��TiO2����ȡ�����ѣ�Ti�����漰���ķ�Ӧ����Ϊ
����þ������Ϊ ����Ar�����еĽ��е������� ��
��4��������ʵ���������ÿ�����þ��Ϊԭ����ȡ��������þ(Mg3N2)����֪��
Mg+H2O MgO+H2�� Mg3N2 +6H2O =3Mg(OH)2+2NH3��
MgO+H2�� Mg3N2 +6H2O =3Mg(OH)2+2NH3��
�ɹ�ѡ���װ�ú�ҩƷ����ͼ��ʾ��
�ٵ��ܿڼ�����Ӵ����ǣ�a ���� ���������������������������� h
��ͨ����Ӧ�ȵ�ȼ ���B����C����װ�õľƾ��ƣ�װ��A�������� ��װ��E�������� ��
�������һ����Сʵ����֤�����ǵ���þ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
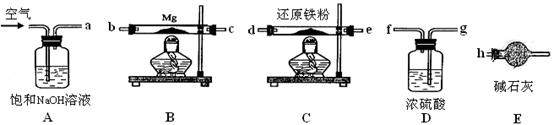
ijͬѧ����ʵ���������Fe��ˮ������Ӧ��ʵ�飬װ����ͼ�ס������֡�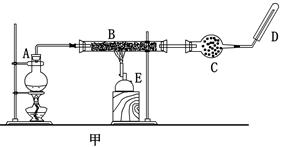
��֪��B�з����������ʯ���Ļ���C�зŵ��Ǹ������EΪ�ƾ���ƣ�GΪ������˿���ֵľ�
���ơ��Ա���װ�ã��ش��������⣺
��1����μ����װ�õ������ԣ� ��
��2����װ����ʪɳ�ӵ������ǣ� ��
��3��B��������Ӧ�Ļ�ѧ����ʽ�� ��
��4����ȡmg����������ʯ����ϣ�Ȼ�������������ų������ռ��������������Ϊ��״��ΪVL�����۵�ת����Ϊ ���г�����ʽ���ɣ����ػ���
��5��Ϊ��֤����Ӧ��Ĺ��������к���+3�۵�Fe����ͬѧȡ��������������Թ��У�����һ����������ʹ���������ܽ⣬���ˣ���������Һ�еμ�KSCN��Һ������۲쵽��Һ����ɫû�仯������˼������ͬѧ��Ϊ��������˵����Ӧ��Ĺ��������в�����+3��Fe�����������ǣ� _��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
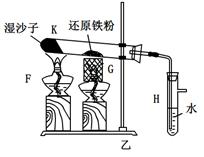
��10�֣�ij�Ȼ�����Ʒ����FeCl2���ʡ���Ҫ�ⶨ������Ԫ�ص�����������ʵ�鰴���²�����У�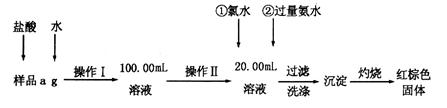
��1������I���õ��IJ����������ձ����������⣬��������________________________�����������ƣ���
��2��д��������ˮ������Ӧ�����ӷ���ʽ____________________��
��3����������Ѿ�ϴ�Ӹɾ��IJ�����������______________________________________
___________________________________________________________________________��
��4����������ΪW1g�����Ⱥ����������ɫ����������ΪW2g������Ʒ����Ԫ�ص�����������____________________���г�ԭʼ��ʽ�����軯��������ȷ�����ղ����Ľ��ƫ�����������ԭ�������_____________________��д��һ��ԭ�ɣ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�˽̰桶����1������Fe3+��Fe2+��ת����ʵ��̽����ijУ��ʦΪ�˼���ѧ�����ÿ��ʵ�����ʱ��Ӧ��������˼·�������һ�ݻ�ѧ̽��ѧϰ������-˼·���ʾ���
��1�������±���������д���ÿһ���������̵�˼·
| ���� | ˼· |
| 1.������裺Fe2+���л�ԭ�ԣ����Ա�������Fe3+��Fe3+���������ԣ����Ա���ԭ��Fe2+ | �ٸ���������ԭ��Ӧԭ����һ�� �����л�ԭ�ԣ� ������������ |
| 2.���ʵ��1��ȡ����FeCl2��Һ���μӼ���H2O2��Һ��������Һ�еμӼ���KSCN��Һ���۲���Һ�Ƿ��Ѫ��ɫ | �������ӷ���ʽ��ʾѡ��H2O2��ԭ�� �������ӷ���ʽ��ʾ��Һ��Ѫ��ɫ��ԭ�� |
| 3.���ʵ��2��ȡ����FeCl3������һ�Թܣ�������е�����ˮ�ܽ⣬�μӼ���KSCN��Һ��Ѹ�ټ����������ۣ������Թ������۲���Һ��Ѫ��ɫ�Ƿ���ȥ | ��ѡ�����۵�ԭ���� ���û�ѧ����ʽ��ʾ�� ��ΪʲôҪ����е�ˮ |
| 4.ʵʩʵ�� | ���� |
| ���� | ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
������ⷨ���Ѱ۲����ķ�Һ[���д���FeSO4��H2SO4������Fe2(SO4)3��TiOSO4 ]���������������Ͳ�Ѫ�������������������������£�
��ش�
��1��������з��������������_______������ޱ������һ������նȣ�ԭ����______________
��2����Һ�е�TiOSO4�ڲ������ˮ����������(��Ҫ�ɷ�ΪTiO2?xH2O)�Ļ�ѧ����ʽΪ_________
_ ������ܵ����ӷ���ʽΪ____________________________________��
��3����ƽ���ƶ�ԭ�����Ͳ�����м������ܵõ�����������ԭ��______________________________��
��4����ƽ���Ը��������Һ������������Һ��Ӧ�����ӷ���ʽ��
_____ Fe2+ + _____ MnO4�� + _____ H+ = _____Fe3+ +_____ Mn2+ +_____
ȡ��������þ�����Ʒa g,����ϡ�������100.00 mL��Һ��ȡ��20. 00 mL��Һ,��KMnO4��Һ�ζ���������KMnO4����Ӧ����������0.1000 mol��L��1 KMnO4��Һ20.00mL�������þ�����FeSO4? 7H2O����������Ϊ���Ժ�a��ʽ�ӱ�ʾ) _____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���������У��������ſ������ռ����ǣ�������
| A��NH3 | B��NO | C��NO2 | D��SO2 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com