
����Ŀ���±�ΪԪ�����ڱ���һ���֣���Ԫ�ط��Ż�ѧʽ��ɸ�С�⡣
IA | ���� | IIIA | IVA | VA | VIA | VIIA | 0 | |
2 | �� | �� | �� | |||||
3 | �� | �� | �� | �� | ||||
4 | �� | �� | �� |
(1)��ѧ��������õ�Ԫ��__________________���ǽ�������ǿ��Ԫ����___________________�����ȶ�����̬�⻯����___________________������������Ӧˮ����������ǿ����_____________________________________________��
(2)��������ǿ�ĵ�����ˮ��Ӧ�����ӷ���ʽΪ______________��
(3)�ۢߢ������Ԫ�صļ����Ӱ뾶�ɴ�С��˳��Ϊ_________________��
(4)�ֱ�д���ޡ��ߺ����γɵ���̬�⻯��Ľṹʽ___________________��
(5)�õ���ʽ��ʾ�ڵ���������γɹ���____________________________��
���𰸡�Ar F HF HClO4 2K+2H2O=2OH-+2K++H2�� r(Cl-)��r(N3-)��r(F-)��r(Mg2+) ![]() ��
��![]()
![]()
![]()
![]()
��������
����Ԫ�������ڱ��е�λ��֪���١���Ԫ�طֱ���Na��K��Mg��Ca��Al��C��O��F��Cl��Br��ArԪ�أ��ݴ˽��
(1)ϡ�����廯ѧ��������ã��⼸��Ԫ���У���ѧ��������õ���ArԪ�أ��ǽ�������ǿ��Ԫ��λ�����ڱ����Ͻ�(ϡ���������)�������⼸��Ԫ���зǽ�������ǿ����FԪ�أ����ȶ�����̬�⻯��ΪHF����O��F�����ۣ�������������Ӧˮ����������ǿ����HClO4��
(2)��������ǿ�ĵ�����K��K��ˮ��Ӧ����KOH��������������Ӧ�����ӷ���ʽΪ2K+2H2O=2OH-+2K++H2����
(3)Cl-��O2-��F-��Mg2+��һ�����Ӳ㣬��O2-��F-��Mg2+�����ӽṹ��ͬ���˵����Խ�����Ӱ뾶ԽС�������Ӱ뾶�ɴ�С��˳��Ϊr(Cl-)��r(N3-)��r(F-)��r(Mg2+)��
(4)C��O��H�γɵļ��⻯��ΪCH4��H2O�����ǵĽṹʽ����Ϊ![]() ��
��![]() ��
��
(5)K��������ΪK2O�������ӻ�����õ���ʽ��ʾ���γɹ���Ϊ![]()
![]()
![]() ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������һ�ֶ�Ԫ���ᣬ��������ԭ�����������ȡ�ijУ����С���ͬѧ�������C2H2������ȡH2C2O42H2O���ش��������⣺
��1�������ͬѧ�Ե�ʯ����Ҫ�ɷ�CaC2������CaS��Ca3P2���ʵȣ�Ϊԭ�ϣ�������ͼװ����ȡC2H2��

����ʯ��ˮ��Ӧ�ܿ죬Ϊ�˼�����Ӧ���ʣ�װ��A�г��ñ���ʳ��ˮ����ˮ֮�⣬�����Բ�ȡ�Ĵ�ʩ��____________________________ ��дһ�ּ��ɣ���
��װ��B�У�NaClO��H2S��PH3 ����Ϊ���ἰ���ᣬ��������ԭΪNaCl������PH3�����������ӷ���ʽΪ________________________��
��2�������ͬѧ�����������ϣ���Hg(NO3)2��������Ũ��������C2H2��ȡH2C2O4��2H2O���Ʊ�װ����ͼ��ʾ��

��װ��D�ж�����ݵ�������______________________��
��װ��D������H2C2O4�Ļ�ѧ����ʽΪ___________________________________��
����װ��D�еõ���Ʒ�����辭��__________________����������ƣ������ˡ�ϴ�Ӽ����
��3����������˲ⶨ�����Ʒ��H2C2O4��2H2O����������ʵ�顣���ǵ�ʵ�鲽�����£�ȷ��ȡm g��Ʒ����ƿ�У���������������ˮ�ܽ⣬�ټ�������ϡ���ᣬȻ����c mol/L����KMnO4����Һ���еζ����յ㣬�����ı���ҺV mL��
���ζ�ʱ������ر���Һʢ���ڵζ�����_____________�����ʽ����ʽ������
���ζ������з�����ɫ���ʿ�ʼ�������ӿ죬�������ܵ�ԭ����______________ ��
����Ʒ��H2C2O4��2H2O����������Ϊ_________________���г��� m��c��V �ı���ʽ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1����������Ϊ���������ں��º����ܱ������г���һ������NO��NH3����һ�������·�����Ӧ��6NO(g)+4NH3(g)![]() 5N2(g)+6H2O(g)��
5N2(g)+6H2O(g)��
����˵���÷�Ӧ�Ѵﵽƽ��״̬�ı�־��___��
a.��Ӧ����v(NH3)=v(N2)
b.������ѹǿ������ʱ��������仯
c.������N2�����ʵ�������������ʱ��������仯
d.������n(NO)��n(NH3)��n(N2)��n(H2O)=6��4��5��6
e.12mol N-H�����ѵ�ͬʱ����5mol N��N��
��ij��ʵ���в��������NO��N2�����ʵ�����ʱ��仯��ͼ��ʾ��ͼ��b���Ӧ�����ʹ�ϵ��v(��)___v(��)��d���Ӧ�����ʹ�ϵ��v(��)___v(��)�������������
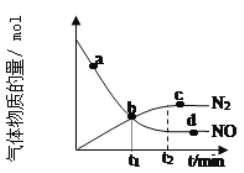
(2)һ�������£���2L�ܱ������ڣ���Ӧ2NO2(g)![]() N2O4(g)��n(NO2)��ʱ��仯���±���
N2O4(g)��n(NO2)��ʱ��仯���±���
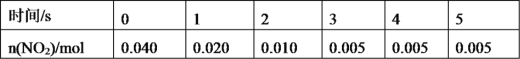
����N2O4��ʾ0��2s�ڸ÷�Ӧ��ƽ������Ϊ___���ڵ�5sʱ��NO2��ת����Ϊ___��
�ڸ����ϱ����Կ��������ŷ�Ӧ���У���Ӧ������С����ԭ����__��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ�о������������Ʋ��������ʵ�飺
a��ij��ȤС�������ͼ��ʾװ�����Ľ��̲�����ͭ�����ᷴӦ��ʵ�飬��̽����ѧʵ�����ɫ����

(1)ʵ��ǰ���رջ��� b���Թ� d �м�ˮ����û�����ܿڣ������Թ� c �� d �Ľ��������� c����Ŀ����__________
(2)�� d �м����� NaOH ��Һ��c �з�һС��ͭƬ���ɷ�Һ©�� a �� c �м���2mL Ũ���ᣬc �з�Ӧ�����ӷ���ʽ___________________________________________������ a �� c �м� 2mL ����ˮ��д�� c �п��ܽ��е�������Ӧ�Ļ�ѧ����ʽ_______________________________________��________________________________________��
b�������£�����Ƭ�ֱ�����������ϡ HNO3 ��Ũ HNO3 ��(��ͼ��ʾ)��

(1)�����������_____________________________________
(2)A����Һ���ձ�Ϊdz��ɫ��������Ӧ�����ӷ���ʽ��______________________
(3)B �������Ա仯����ͬѧ��һ��̽�����£�����������������Ũ�����Ӧ����_______________________
����ʵ�飺�� B �е���Ƭȡ����ϴ������ CuSO4 ��Һ�У������Ա仯�� ��˵��������_____________(����������������������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͭƬ���� 115mL ijŨ�ȵ�Ũ�����У��ڼ��������·�Ӧ����ͭƬȫ���ܽ������Һϡ�͵�500mL���ټ�������п�ۣ�ʹ֮��ַ�Ӧ���ռ��� 2.24L(��״����)���������˲������壬�������������������� 7.5g����ԭ��������ʵ���Ũ��________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������Ԫ�����ڱ���Ԫ�������ɣ��ж�������������ȷ����

A. ��̬�⻯����ȶ��ԣ�H2O��NH3��SiH4
B. ��Ԫ��������Ԫ�ؿ��γɹ��ۻ���������ӻ�����
C. ��ͼ��ʾʵ���֤��Ԫ�صķǽ����ԣ�Cl��C��Si
D. ��������![]() ������o�������ĵ�118��Ԫ�������ڱ���λ�ڵ�������0��
������o�������ĵ�118��Ԫ�������ڱ���λ�ڵ�������0��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���±���Ԫ�����ڱ���һ���֣��ش���ص����⡣
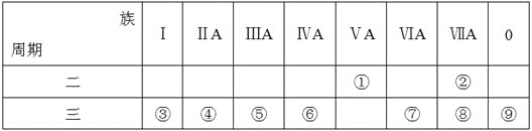
��1��д���ܵ�Ԫ�ط���__��
��2������ЩԪ���У�����õĽ���Ԫ����ˮ��Ӧ�����ӷ���ʽ��__��
��3������ЩԪ���У�����������ˮ����������ǿ����__(����Ӧ��ѧʽ����ͬ)��������ǿ����__��
��4����ЩԪ����(������)��ԭ�Ӱ뾶��С����__(��Ԫ�ط��ţ���ͬ)��ԭ�Ӱ뾶������__��
��5���ڵĵ�����۵�����������ˮ�������Һ��Ӧ�������֮һ��OX2��(O��X�ֱ��ʾ���͢ڵ�Ԫ�ط��ţ���OX2�����û�ѧʽ)���÷�Ӧ�����ӷ���ʽΪ(����ʽ���þ���Ԫ�ط��ű�ʾ)__��
��6���ߵĵͼ�������ͨ������Ba(NO3)2��Һ�е����ӷ���ʽ__��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʵ�鷽���У��ܴﵽ��Ӧʵ��Ŀ�ĵ���
A.���������ڷ���I2��NH4I����
B.���������ڱȽ�CuSO4��MnO2�Ĵ�����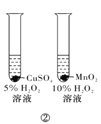
C.���������ڲⶨ�к���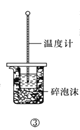
D.���������ڱȽϴ�������������ǿ��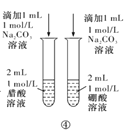
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����̼������ָ�ڿɳ�����չ����ָ���£������ܵؼ���ú̿��ʯ�͵ȸ�̼��Դ���ģ��������������ŷţ��ﵽ������ᷢչ����̬��������˫Ӯ��һ�־��÷�չ��̬���������й�̼Ԫ�ص����ת�����ش��������⣺
��1����֪�״���һ�����ȼ�ϣ��Ʊ��״���úҺ������Ҫ��������֪H2(g)��CO(g)��CH3OH(l)��ȼ���ȷֱ�ΪH����285.8kJ/mol����H����283.0kJ/mol����H����726.5kJ/mol��CO(g)��2H2(g)![]() CH3OH(l) ��H��________ kJ/mol��
CH3OH(l) ��H��________ kJ/mol��
��2��һ���¶��£�һ��������״��ϳ����ʵĴ�ʩ����______��
a.������ʼͨ��![]() ֵ
ֵ
b.���º��ݣ���ͨ�뺤��
c.ʹ�����͵ĸ�Ч������
d.�������ļ״���ʱ����
e.ѹ�����������ѹǿ
��3���ں��º��������£�����˵�������ж���ӦCO(g)��2H2(g) ![]() CH3OH(g)�Ѿ��ﵽƽ��״̬������______��
CH3OH(g)�Ѿ��ﵽƽ��״̬������______��
a.��ϵ��̼�ⵥ����Ŀ���ٸı�
b.��ϵ��n(CO)��n(H2)���ٸı�
c.��ϵ��ѹǿ����ƽ��Ħ���������ٸı�
d.��λʱ��������������CH3OH�����ʵ������
��4���ں�ѹ�������У�����X��Y��Z�ֱ��ʾ��T1��C��T2��C��T3��C�����¶��ºϳɼ״�����Ĺ��̡����Ʋ�ͬ��ԭ��Ͷ�ϱȣ�CO��ƽ�nת������ͼ��ʾ��
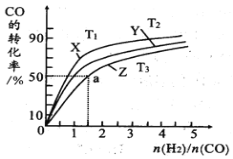
���¶�T1��C��T2��C��T3��C�ɸߵ��͵����Ϊ��_________________��
�����¶�ΪT3��Cʱ����ϵѹǿ����50aMPa����ʼ��Ӧ��Ͷ�ϱ�n(H2)/n(CO)��1.5����ƽ��ʱCO��CH3OH�ķ�ѹ֮��Ϊ__________���÷�Ӧ��ѹǿƽ�ⳣ��Kp�ļ���ʽΪ__________��(Kp�������ѹ�ݵij˻��뷴Ӧ���ѹ�ݵij˻��ı�ֵ��ij���ʵķ�ѹ������ѹǿ�������ʵ����ʵ�������)��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com