
̼��þ������һ�����͵��������β����е���ǿ���ϡ�
��1���ϳɸ����ʵIJ������£�
����1������0.5mol��L-1MgSO4��Һ��0.5mol��L-1NH4HCO3��Һ��
����2������Ͳ��ȡ500mL NH4HCO3��Һ��1000mL�Ŀ���ƿ�У��������������¶ȿ�����50�档
����3����250mL MgSO4��Һ��μ���NH4HCO3��Һ�У�1min�ڵμ�����ð�ˮ������ҺpH��9.5��
����4������1h���ˣ�ϴ�ӡ�
����5����40�����ո������и���10h����̼��þ�����Ʒ��MgCO3��nH2O n=1~5����
�ٲ���2�����¶���50�棬�Ϻõļ��ȷ����� ��
�ڲ���3����MgCO3��nH2O���������ӷ���ʽΪ ��
�۲���4�����Ƿ�ϴ�Ӹɾ��ķ����� ��
��2���ⶨ�ϳɵ�MgCO3��nH2O�е�nֵ��
����1.000g̼��þ���룬������ͼ��ʾ�Ĺ��ƿ�м���ˮ����ϡ�����뾧�뷴Ӧ�����ɵ�CO2��NaOH��Һ���գ��������·�Ӧ4~5h����Ӧ���ڽ��¶�����30�棬�����ձ��е���Һ����֪Ũ�ȵ�����ζ������CO2���������ظ���������2�Ρ�
��ͼ������������� ��
��������Ӧ����Ҫ���µ�30�棬��ҪĿ���� ��
����3��ʵ����ÿ1.000g̼��þ���������CO2ƽ��ֵΪa mol����nֵΪ ���ú�a�ı���ʽ��ʾ����
��3����ȡ100g���������Ʒ�������ط���������������ͼ��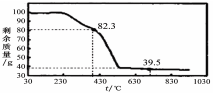
��������ºϳɵľ����У�n= ��ѡ�1��2��3��4��5����
��1����ˮԡ���� ��Mg2++HCO3-+NH3��H2O+(n-1)H2O=MgCO3��nH2O+NH4+
��ȡ���һ��ϴ��Һ���������������ữ��BaCl2��Һ���������ֳ�������ϴ�Ӹɾ�
��2����ƽ��ѹǿ
���¶����������ܽ�ȼ�С��ʹ���ƿˮ���ܽ�Ķ�����̼�����ݳ����������Ƴ�����գ�
�ۣ�1.000-84a��/18a
��3��1
���������������1����ˮԡ�������Ⱦ��ȣ����ڿ��ƣ�������100����ɲ���ˮԡ���ȣ���Mg2++HCO3-+NH3��H2O+(n-1)H2O=MgCO3��nH2O+NH4+ �۳��������в��������ͨ��ϴ��Һ�к��е�����������SO42-���ж��Ƿ�ϴ�Ӹɾ�����2���ٷ�Ӧ��ϵ�������壬��ֹѹǿ������Σ�գ���������ã����¶����������ܽ�ȼ�С��ʹ���ƿˮ���ܽ�Ķ�����̼�����ݳ����������Ƴ�����գ�
��n(MgCO3)=n(CO2)="a" mol
m(H2O)=(1.000-84a)g
n(H2O)=��1.000-84a��/18 mol
��n=��1.000-84a��/18a
��3����ͼ�п��Եó�MgCO3��nH2O���82.3gʱΪʧȥ�ᾧˮ�ķ�Ӧ�����õ�����MgO��
100��18n/��84+18n��=100-82.3
n=1
���㣺��������ɵIJⶨΪ���壬���黯ѧʵ����������ѡ��ϴ�Ӳ���������������ʵ�鷽������������۵��й����⡣



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij�о�С�������KMnO4�ⶨFeSO4�ĺ�����
(1)��ʵ��ǰ������Ҫ��ȷ����һ�����ʵ���Ũ�ȵ�KMnO4��Һ200 mL������ʱ��Ҫ����������ƽ��ҩ���⣬����Ҫ�������Уߣߣߣߣߡ��ߣߣߣߣߡ��ߣߣߣߣߡ��ߣߣߣߣߡ��ߣߣߣߣߡ�
����KMnO4(�ữ)�ζ�ʱ����������������Һ���ڣߣߣߣߣ�(������)�У����������Һ����______(������)�У��ζ��յ�ʱ��Һ����ɫΪ�ߣߣߣߣ�ɫ��
(2)��һ�о�С������������²���������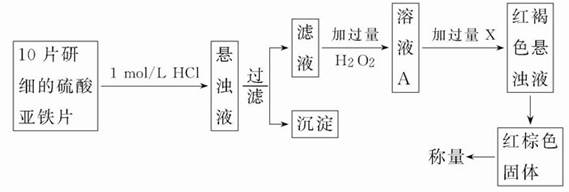
�ٹ���ʱ�õ��IJ��������Уߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
�ڴӺ��ɫ������Һ�����ij����������������Ļ��������Уߣߣߣߣ�(��������˳����д)��
A���ˣ�Bϴ�ӣ�C��ȡ��D��Һ��E��ȴ��F����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
(15��)��ʽ̼��ͭ�ijɷ��ж��֣��仯ѧʽһ��ɱ�ʾΪxCu(OH)2��yCuCO3��
(1)��ȸʯ����ɫ����һ������ı�ʯ������Ҫ�ɷ���Cu(OH)2��CuCO3��ij��ȤС��Ϊ̽����ȡ��ȸʯ����ѷ�Ӧ���������������ʵ�飺
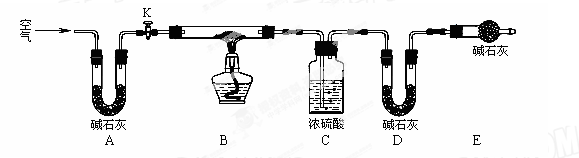
ʵ��1����2.0mL 0.50 mol��L�C1��Cu(NO3)2��Һ��2.0mL 0.50 mol��L�C1��NaOH��Һ��0.25 mol��L�C1��Na2CO3��Һ��������ʾ�����ϡ�
ʵ��2�������ʱ����Ļ�����ڱ�����ʾ�¶��·�Ӧ��
ʵ���¼���£�
���� ����
| ��� | V (Na2CO3)/mL | ������� | | ��� | ��Ӧ�¶�/�� | ������� |
| 1 | 2.8 | �ࡢ��ɫ | | 1 | 40 | �ࡢ��ɫ |
| 2 | 2.4 | �ࡢ��ɫ | | 2 | 60 | �١�dz��ɫ |
| 3 | 2.0 | �϶ࡢ��ɫ | | 3 | 75 | �϶ࡢ��ɫ |
| 4 | 1.6 | ���١���ɫ | | 4 | 80 | �϶ࡢ��ɫ(������ɫ) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��������ƣ�Na2S2O3����������һϵ�з�Ӧ�Ƶã�
��Na2CO3+SO2 =Na2SO3+CO2
��Na2S+SO2+H2O=Na2SO3+H2S
��2H2S+SO2=3S��+2H2O
��Na2SO3 + S  Na2S2O3��
Na2S2O3��
��������Һ����������ΪNa2S2O3?5H2O��Na2S2O3��5H2O��40��45���ۻ���48��ֽ⣻
Na2S2O3������ˮ���������Ҵ�����ˮ���й����ʵ��ܽ��������ͼ��ʾ��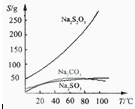
���ְ����·����Ʊ�Na2S2O3��5H2O��
�����ƺ�̼���ư���ӦҪ�����һ������������ƿ�У�ע��150mL����ˮʹ���ܽ⣬�ڷ�Һ©���У�ע��Ũ���ᣬ��װ��2�м����������ƹ��壬������ͼ��װ��װ�á�
���ʣ�����2������Ϊ ��
װ��6�пɷ��� ��
| A��BaCl2��Һ | B��ŨH2SO4 |
| C������KMnO4��Һ | D��NaOH��Һ |

 6I-+Cr2O72-+14H+=3I2+2Cr3++7H2O���ټ��뼸�ε�����Һ������������Na2S2O3��Һ�ζ���������Ӧ�� I2+2S2O32-=2I-+S4O62-���ζ��յ������Ϊ ��
6I-+Cr2O72-+14H+=3I2+2Cr3++7H2O���ټ��뼸�ε�����Һ������������Na2S2O3��Һ�ζ���������Ӧ�� I2+2S2O32-=2I-+S4O62-���ζ��յ������Ϊ ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij�о���ѧϰС������ữ����������Һ����I�����ڵĺ����Բ�������Ȥ��ͬѧ�Ǹ��ݱ�������˼��������·�����Ʋ�������ʵ��̽����
[�������]
����1�����ɵ�AgI������ˮ�����ܱ�HNO3������
����2��HNO3�������ԣ��ܽ�I��������I2��
[���ʵ�鷽������֤����]
��1����ͬѧ��KI��Һ�еμ����ữ��AgNO3��Һ�����л�ɫ�������ɡ���֤�˼���1��������д���йػ�ѧ����ʽ ��
��2����ͬѧ���ʵ����֤2�����������±������ݡ�
| ʵ�鲽�裨��Ҫ��д����������̣� | Ԥ������ͽ��� |
| | ����Һ����������2������ ����Һ������������2�������� |
| ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijͬѧ��������̽��ʱ��������1��0molL-1Ba(OH)2��Һ����ֻ�ҵ��ڿ����б�¶�Ѿõ�Ba(OH)2��8H2O�Լ�����ѧʽ����315������������������Һʱ������ȡ�Լ���ˮ�н������ܽ⣬�ձ��д��ڴ���δ���
Ϊ̽��ԭ��ͬѧ���Ba(OH)2��8H2O �����ܽ�����ݣ����±���
| �¶� | 283K | 293K | 303K |
| �ܽ�ȣ�g/100g H2O�� | 2��5 | 3��9 | 5��6 |
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1��ȡ�����Լ��ڽྻ�ձ��У�������������ˮ����ֽ��裬���ã����ˣ�����Һ�ͳ����� | ������������ |
| ����2��ȡ������Һ���Թ��У��μ�ϡ���ᡣ | _______________________ |
| ����3��ȡ��������1�еij������Թ��У� ,���Ӵ��������ܽ����������嵼�����ʯ��ˮ�С� | _______________________ ______________________ |
| ����4��ȡ����1�е���Һ���ձ���,______________________________ | _______________________,˵�����Լ��к���Ba(OH)2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��̼���ƺ����ᷴӦ�Ļ�ѧ����ʽΪ��2Na2CO4+4HCl=4NaCl+2CO2��+O2��+2H2O����Ʒ��̼������һ�㶼����Na2CO3��Ϊ�˲ⶨ���Ĵ��ȣ�ȡһ��������Ʒ�����ᷴӦ��ͨ��������������������������Լ������̼���Ƶĺ�����
��1��������ͼ�ṩ������װ�ã���װһ�ײⶨ��Ʒ��̼���Ƶ�ʵ��װ�ã���Щװ�õ�����˳���ǣ���ӿ���ĸ���� ��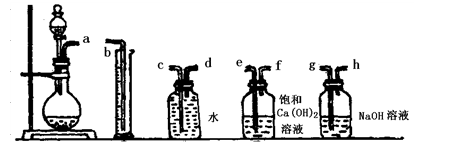
�� �� �� �� ��
��2��װ�âܵ������� ��
��3�����ʵ��ʱ����ȡw g��Ʒ�������ᷴӦ����������������״����ΪV mL�������Ʒ�Ĵ���Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�ᴿ�������ʣ������ڵ����������ʣ�����ѡ�õij����Լ��ͷ��뷽������ȷ����(����)
| | ���ᴿ������ | �����Լ� | ���뷽�� |
| A | �������������ᣩ | CCl4 | ��ȡ����Һ |
| B | ����(��ϩ) | ����KMnO4��Һ | ϴ�� |
| C | �屽���壩 | ����������Һ | ��Һ |
| D | �������ӣ� | Ũ��ˮ | ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�� ��CH3CH2OH��CH3CH2Br��NH4Cl��Һ������ɫҺ�壬ֻ��һ���Լ����ܰ� ���Ǽ��������Լ���(����)
��CH3CH2OH��CH3CH2Br��NH4Cl��Һ������ɫҺ�壬ֻ��һ���Լ����ܰ� ���Ǽ��������Լ���(����)
| A����ˮ���������������� | B��NaOH��Һ |
| C��Na2SO4��Һ | D��Br2��CCl4��Һ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com