
��12�֣��밴ָ��Ҫ������������⡣
�������Ը��������Һ��ͨ������������壬������ر���ԭΪ�����̣�����д���ӷ���ʽ��___________________��
������������������Һ�еμӹ���˫��ˮ������д���ӷ���ʽ��_____________________��
�����廯������Һ��ͨ������ʵ������������������ӷ���ʽ��__________________��
�Ƚ��������������ڹ�����ϡ���ᣨ����д���ӷ���ʽ��__________________________��
���Ҵ��������ữ���ظ������Һ���������ᣬ�ظ���ر���ԭΪ�����������ɻ�ѧ����ʽ��_______________��
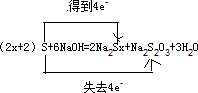
��S + NaOH = Na2Sx + Na2S2O3 + H2O (�ú�x�Ĵ���ʽ��ƽ����ʽ�����������ת�Ƶķ������Ŀ)_________________________________________________________________
 ѧ���쳵�����ּ��ں�����ҵϵ�д�
ѧ���쳵�����ּ��ں�����ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013ѧ�������ʡ������ѧ�ھ����¿���ѧ�Ծ��������棩 ���ͣ������
��12�֣��밴ָ��Ҫ������������⡣
�������Ը��������Һ��ͨ������������壬������ر���ԭΪ�����̣�����д���ӷ���ʽ��___________________��
������������������Һ�еμӹ���˫��ˮ������д���ӷ���ʽ��_____________________��
�����廯������Һ��ͨ������ʵ������������������ӷ���ʽ��__________________��
�Ƚ��������������ڹ�����ϡ���ᣨ����д���ӷ���ʽ��__________________________��
���Ҵ��������ữ���ظ������Һ���������ᣬ�ظ���ر���ԭΪ�����������ɻ�ѧ����ʽ��_______________��
��S + NaOH = Na2Sx + Na2S2O3 + H2O (�ú�x�Ĵ���ʽ��ƽ����ʽ�����������ת�Ƶķ������Ŀ)_________________________________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�������ʡ���������и������ϣ��¿���ѧ�Ծ���9�·ݣ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com